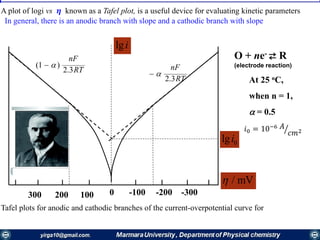
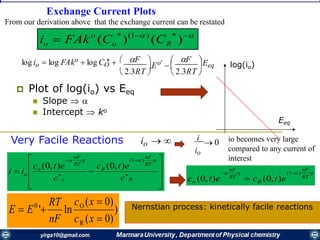
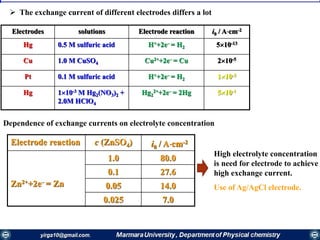
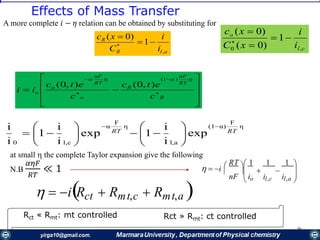
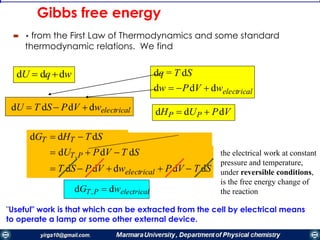
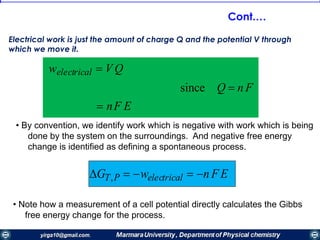
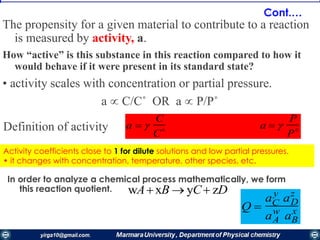
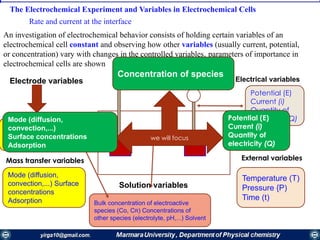
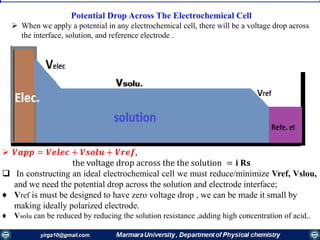
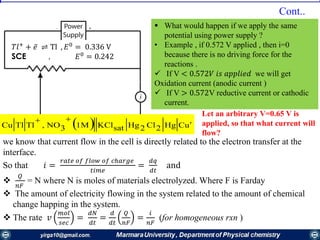
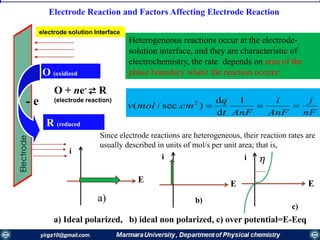
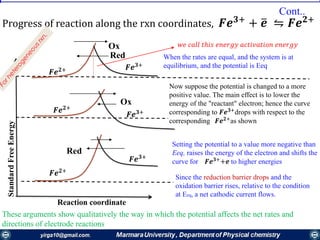
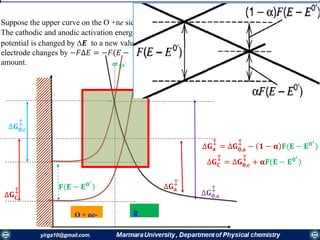
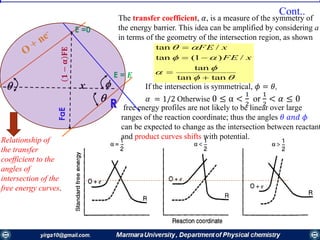
The document covers the fundamental principles of electrochemistry, focusing on redox reactions, electrode-solution interfaces, and the thermodynamics of electrochemical cells. It discusses the Nernst equation, Gibbs free energy, mass transfer processes, and factors affecting electrode reactions, including potential drops and overpotentials. Additionally, it emphasizes the relationship between current, voltage, and the rates of electrochemical reactions, highlighting the kinetics and transport phenomena that govern these processes.

![ΔG°
∆𝑯 𝟎
, ∆𝑺 𝟎
Electrochemical
celldata𝑬𝟎
𝒄𝒆𝒍𝒍
Equilibrium
constantan
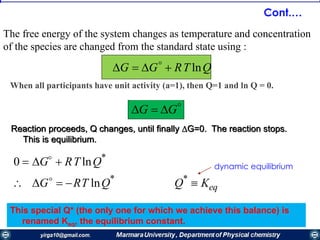
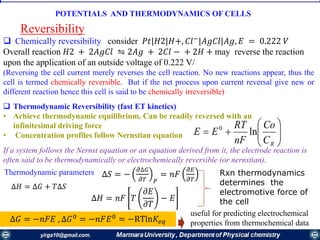
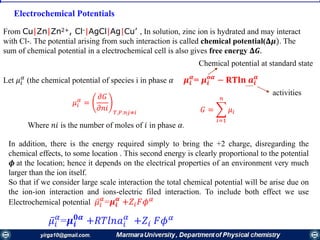
ΔG° = -nFE°cell
Reaction Parameters at the Standard State
ΔG° Q E°cell
Reaction at standard-state
conditions
< 0 > 1 > 0 spontaneous
= 0 =1 = 0 at equilibrium
> 0 < 1 < 0 nonspontaneous
• When Q < 1, [reactant] > [product], ln Q < 0, so Ecell > E°cell
• When Q = 1, [reactant] = [product], ln Q = 0, so Ecell = E°cell
• When Q > 1, [reactant] < [product], ln Q > 0, so Ecell < E°cell
Ecell = E°cell - ln Q
RT
nF
Summery of interrelationship of G°, E°cell, and Q.
Cont.…](https://image.slidesharecdn.com/electrochemistrypresentaion1-171025001847/85/Electrochemistry-9-320.jpg)
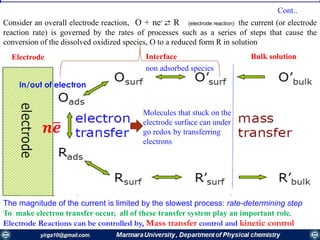

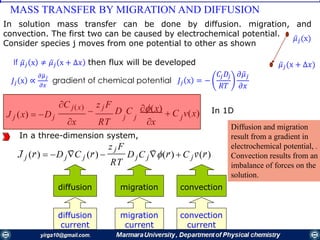
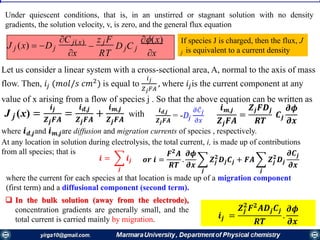
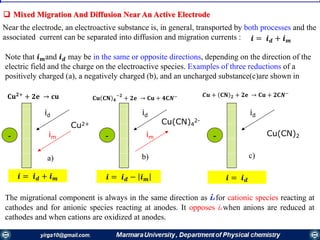
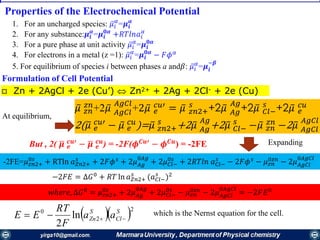
![Mass transfer to an electrode is governed by the Nernst-Planck equation, written for one-
dimensional mass transfer along the x-axis as
𝑱𝒊 𝒙 = −𝑫𝒊
𝝏𝑪𝒊 𝒙
𝝏 𝒙
−
𝒛𝒊 𝑭
𝑹𝑻
𝑫𝒊 𝑪𝒊
𝝏∅ 𝒙
𝝏𝒙
+ 𝑪𝒊 𝒗 𝒙
where Ji(x) is the flux of species i (mol /s/cm2) at distance x from the surface, Di is
the diffusion coefficient (cm2/s),
𝝏𝑪 𝒊 𝒙
𝝏 𝒙
is the concentration gradient at distance x,
𝝏∅ 𝒙
𝝏𝒙
is the potential gradient, zi and Ci are the charge (dimensionless) and
concentration (mol cm-3) of species i, respectively, and v(x) is the velocity (cm/s) with
which a volume element in solution moves along the axis.
diffusion, migration, and convection,
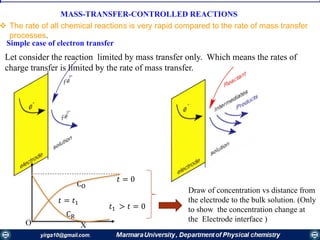
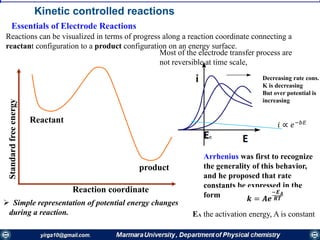
If the mass transfer is the slowest step of the electrode reaction, then the electrode reaction is
termed as being “electrochemically reversible”. At each potential difference (E) of the
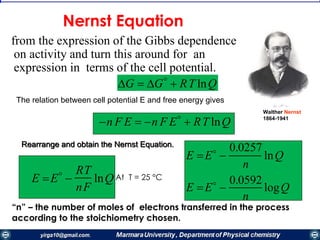
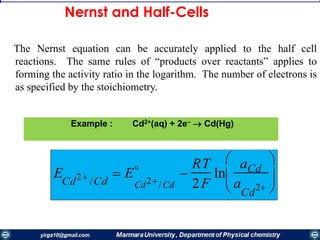
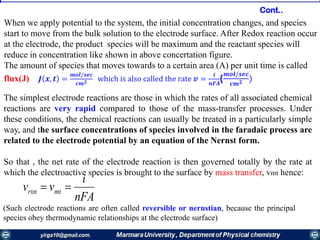
interface, the electrode reaction is in redox equilibrium, which is described by the Nernst equation:
0x
0x'0
[R]
[O]
ln
nF
RT
EE
)(equlibrumat1
),(
,
E
0
txC
txC
R
O
Cont..](https://image.slidesharecdn.com/electrochemistrypresentaion1-171025001847/85/Electrochemistry-19-320.jpg)
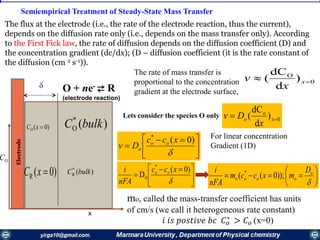
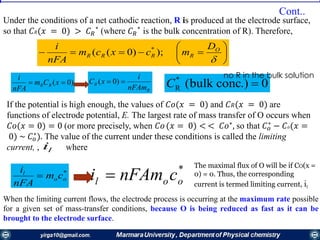
![))0(( *
xccm
nFA
i
ooo
*
ool cnFAmi o
l
o
nFAm
ii
xc
)0(
Thus, the concentration of species О at the
electrode surface is linearly related to the
current and varies from 𝐶 𝑜
∗
, when i = 0, to a
negligible value, when i = 𝑖𝑙.
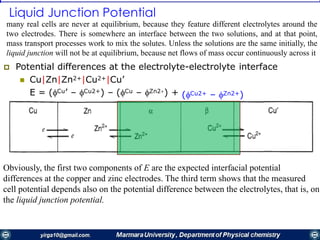
If the kinetics of electron transfer are rapid, the concentrations of О and R at the electrode
surface can be assumed to be at equilibrium with the electrode potential, as governed
by the Nernst equation for the half-reaction
0x
0x'0
[R]
[O]
ln
nF
RT
EE Such a process is called a nernstian reaction
We can derive the steady-state i-E curves for nernstian reactions under several different conditions.
I. R Initially Absent
When 𝐶 𝑅
∗
=0, can be obtained
R
R
nFAm
i
xc )0(
o
l
nFAm
ii
xc
)0(0
)0(
)0(
ln
R
O'0
xc
xc
nF
RT
EE a)(..........lnln'0
i
ii
nF
RT
m
m
nF
RT
EE l
O
R
)0( xCR
Using this, and
Cont..](https://image.slidesharecdn.com/electrochemistrypresentaion1-171025001847/85/Electrochemistry-22-320.jpg)
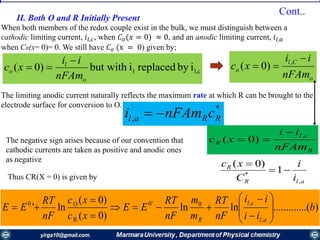
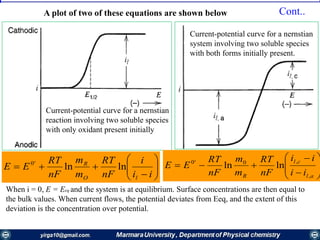
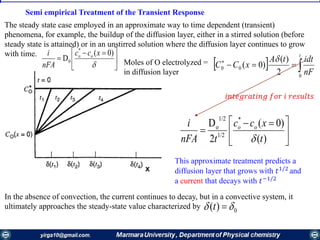

![nFA
i
ck c
Off
nFA
i
ck a
Rbb
nFA
i
ckck Rbfbfnet 0
][ RbOfac ckcknFAiii
)](exp[ '00
EE
RT
nF
kkf )]()1exp[( '00
EE
RT
nF
kkb
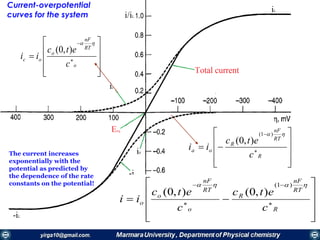
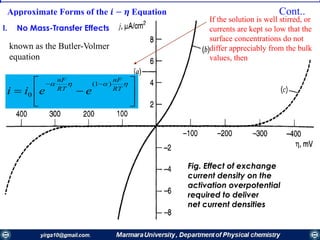
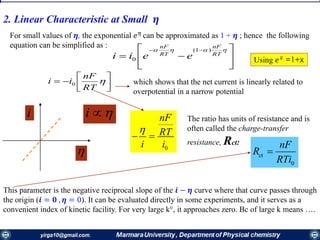
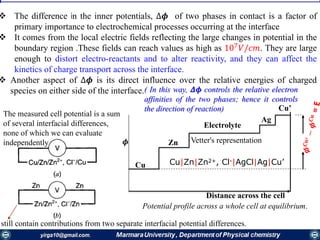
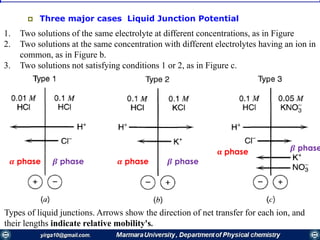
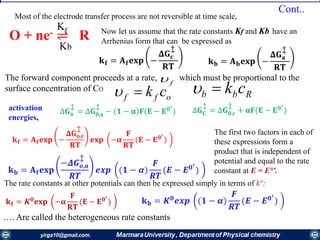
Rate constants depend on the potential! The unique feature of electrochemical rate constants.
Thus, the rate of the electrode reaction can be controlled by the potential!
overall current, i[A], can be viewed as the
difference of the cathodic (reduction)
current, ic [A], and the anodic (oxidation)
current, ia [A]:
ac iii
Each of the currents is proportional to their
corresponding heterogeneous rate
constant
t)(0,CFAk
t)(0,CFAk
Rba
ofC
i
i
Net current:
][
][
O
R
K
k
k
b
f
BUTLER-VOLMER MODEL FOR THE ONE-STEP, ONE-ELECTRON PROCESS
O
Rai
ci
O + ne- ⇌ R
Kf
Kb](https://image.slidesharecdn.com/electrochemistrypresentaion1-171025001847/85/Electrochemistry-43-320.jpg)
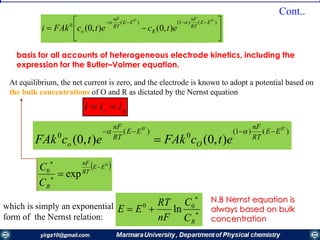
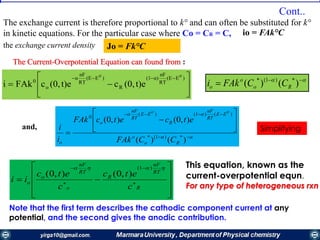
,0(FAk0
tCi Oo
)(
0
'0
EE
RT
nF
oo eCFAki
raised to the -a power, 0
exp0
EE
RT
nF
RC
C
0
0
EE
RT
nF
e
C
C
R
)()( )1(
Ro
o
o CCFAki
At equilibrium, the net current is zero, and the electrode is known to adopt a
potential based on the bulk concentrations of О and R as dictated by the Nernst
equation.
Cont..](https://image.slidesharecdn.com/electrochemistrypresentaion1-171025001847/85/Electrochemistry-45-320.jpg)