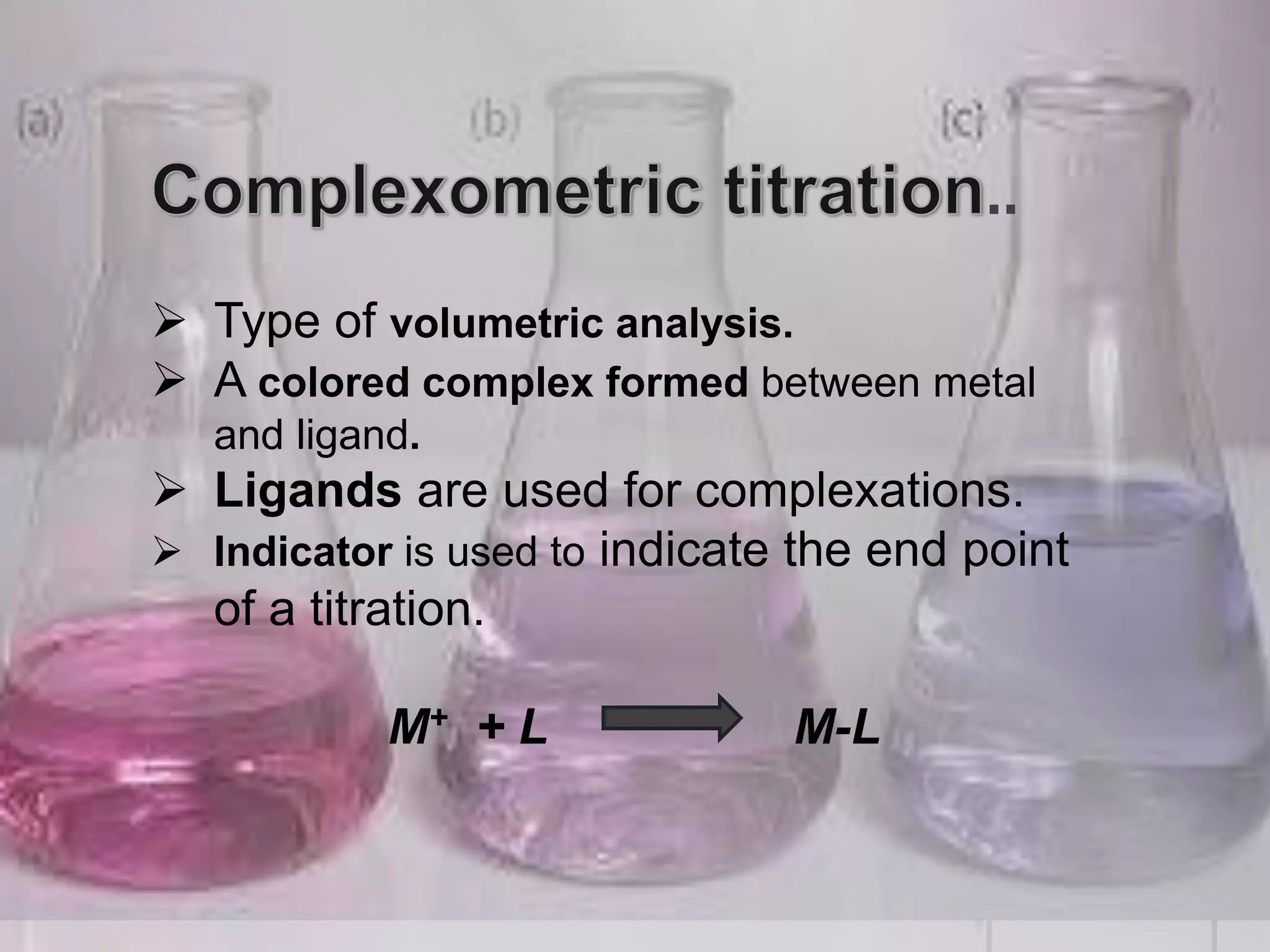
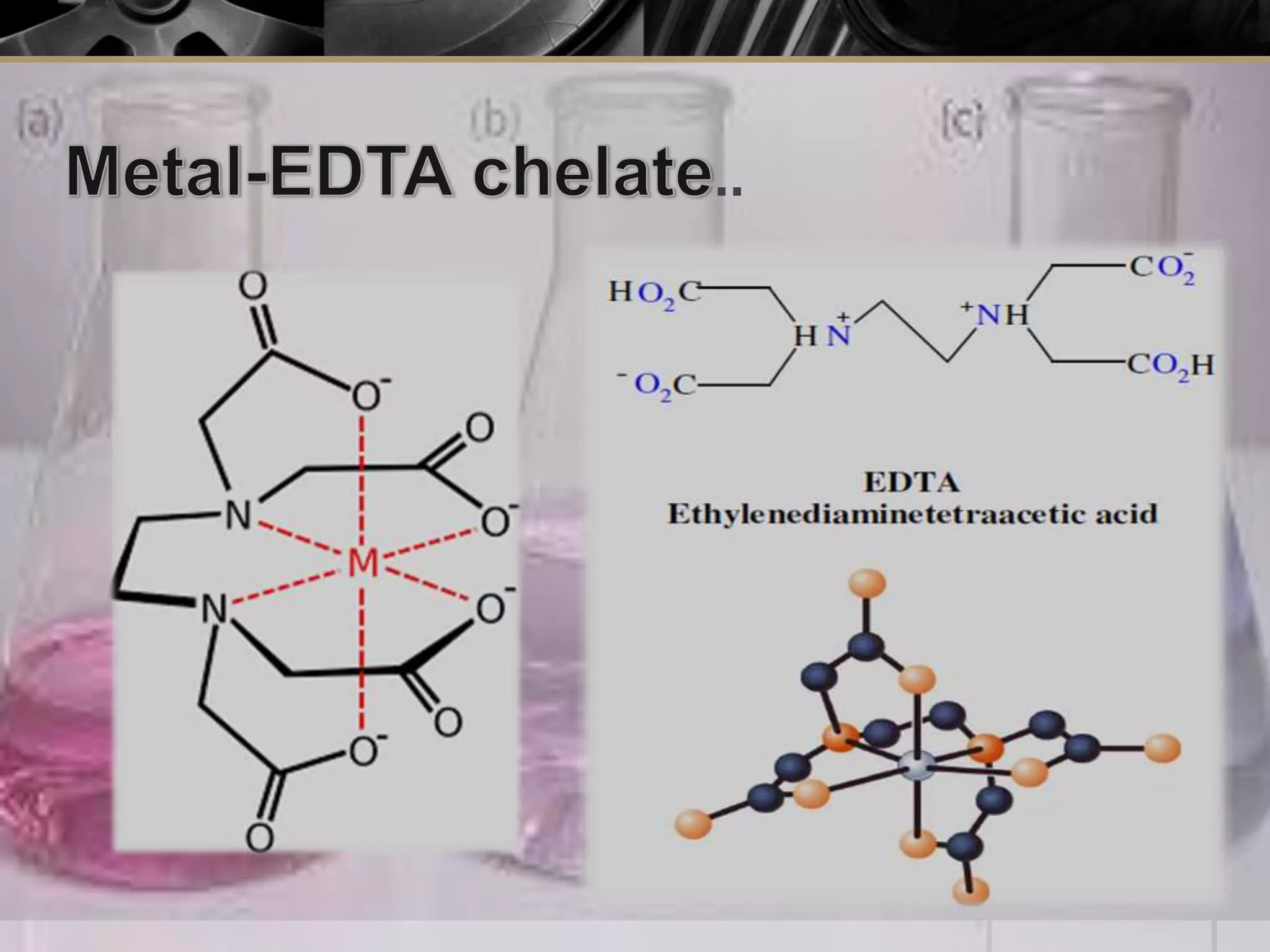
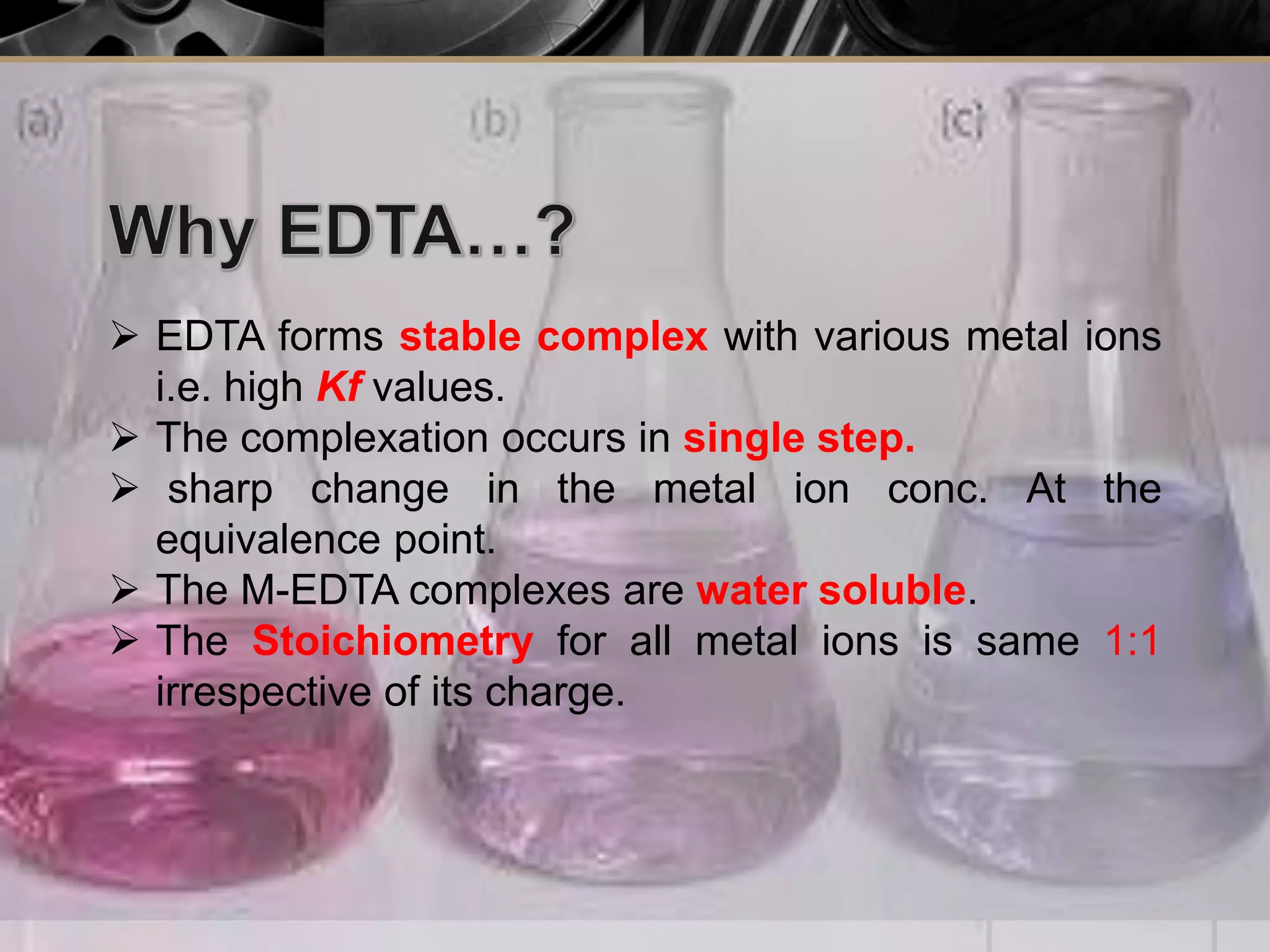
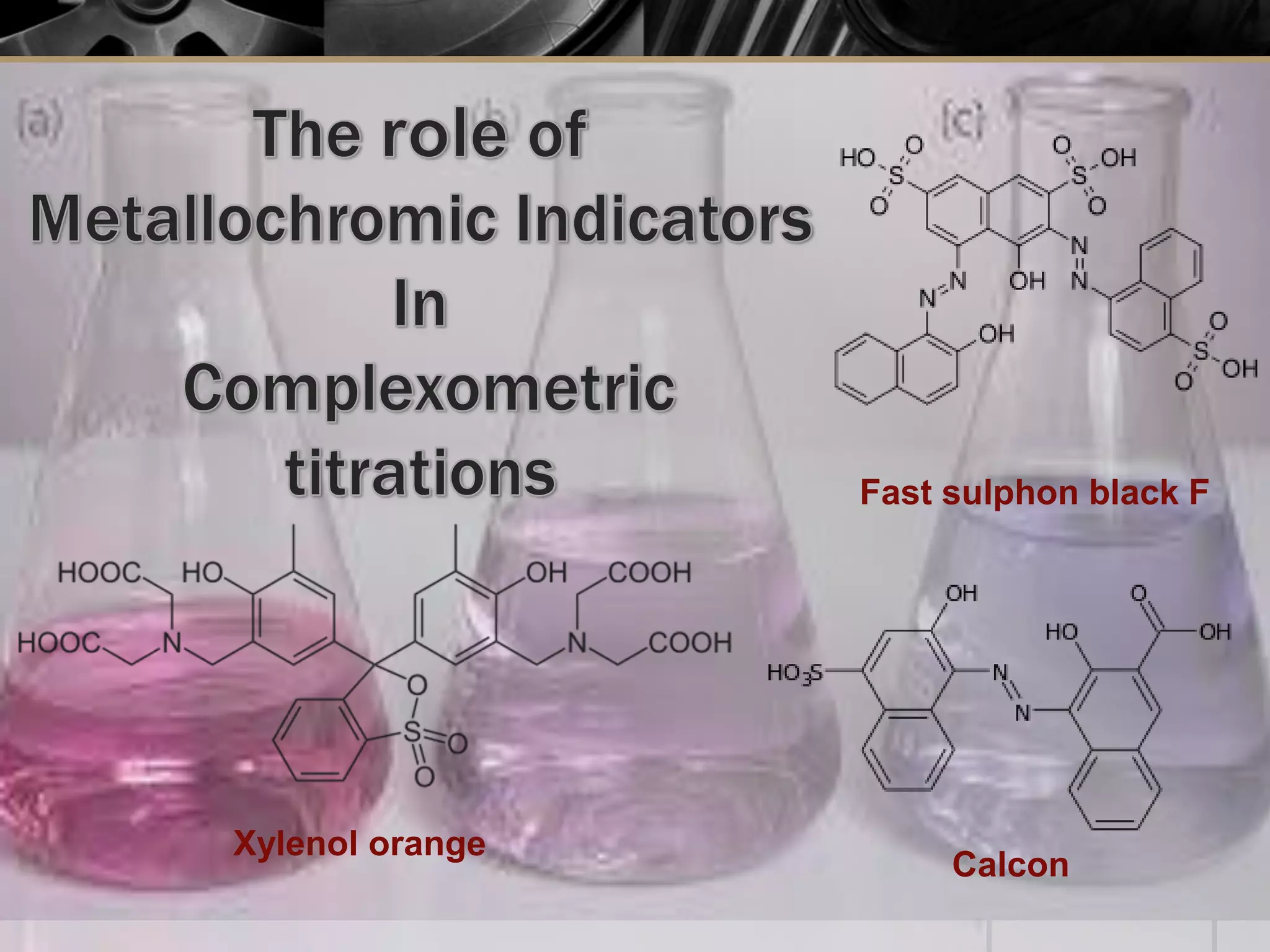
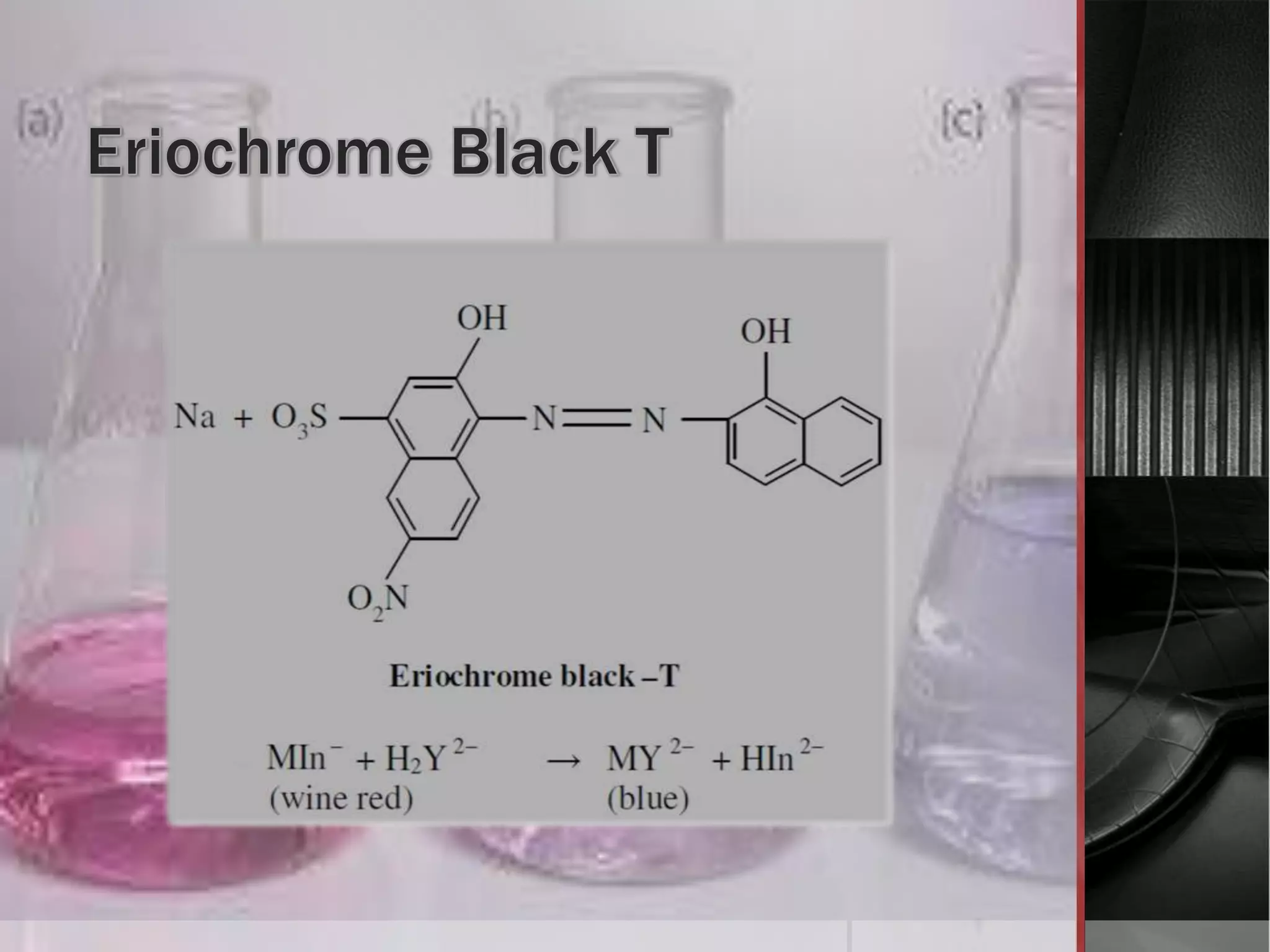
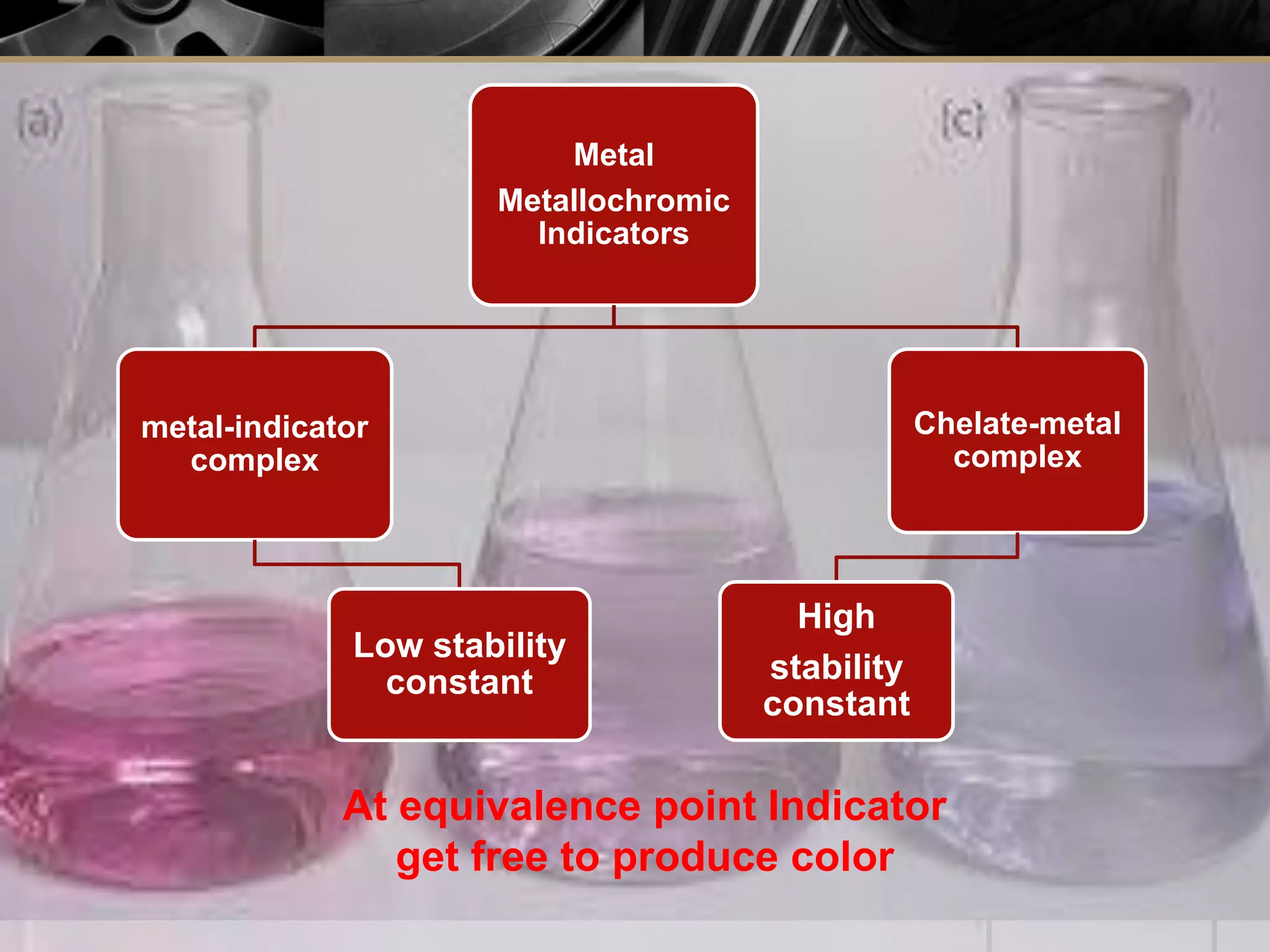
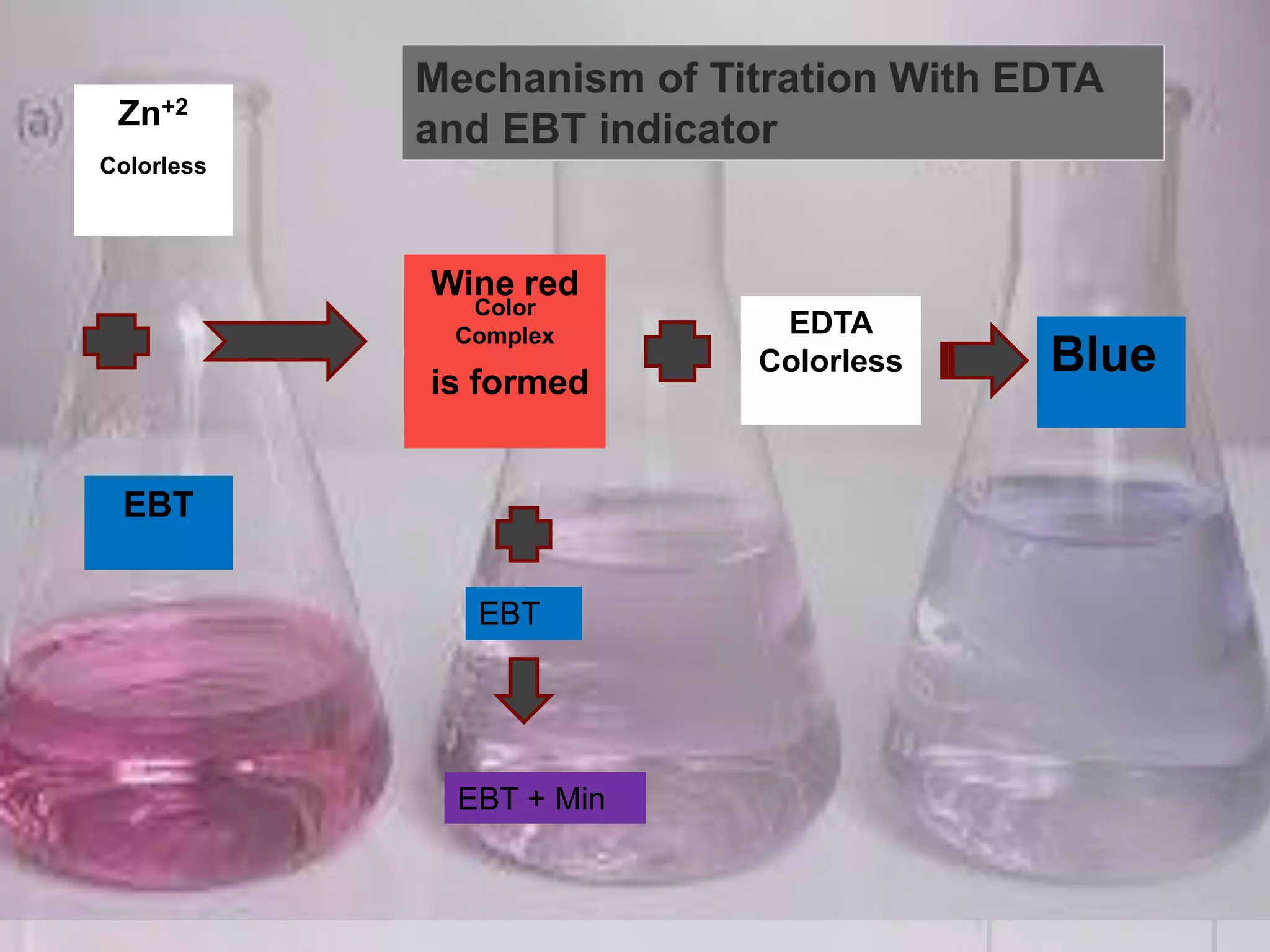
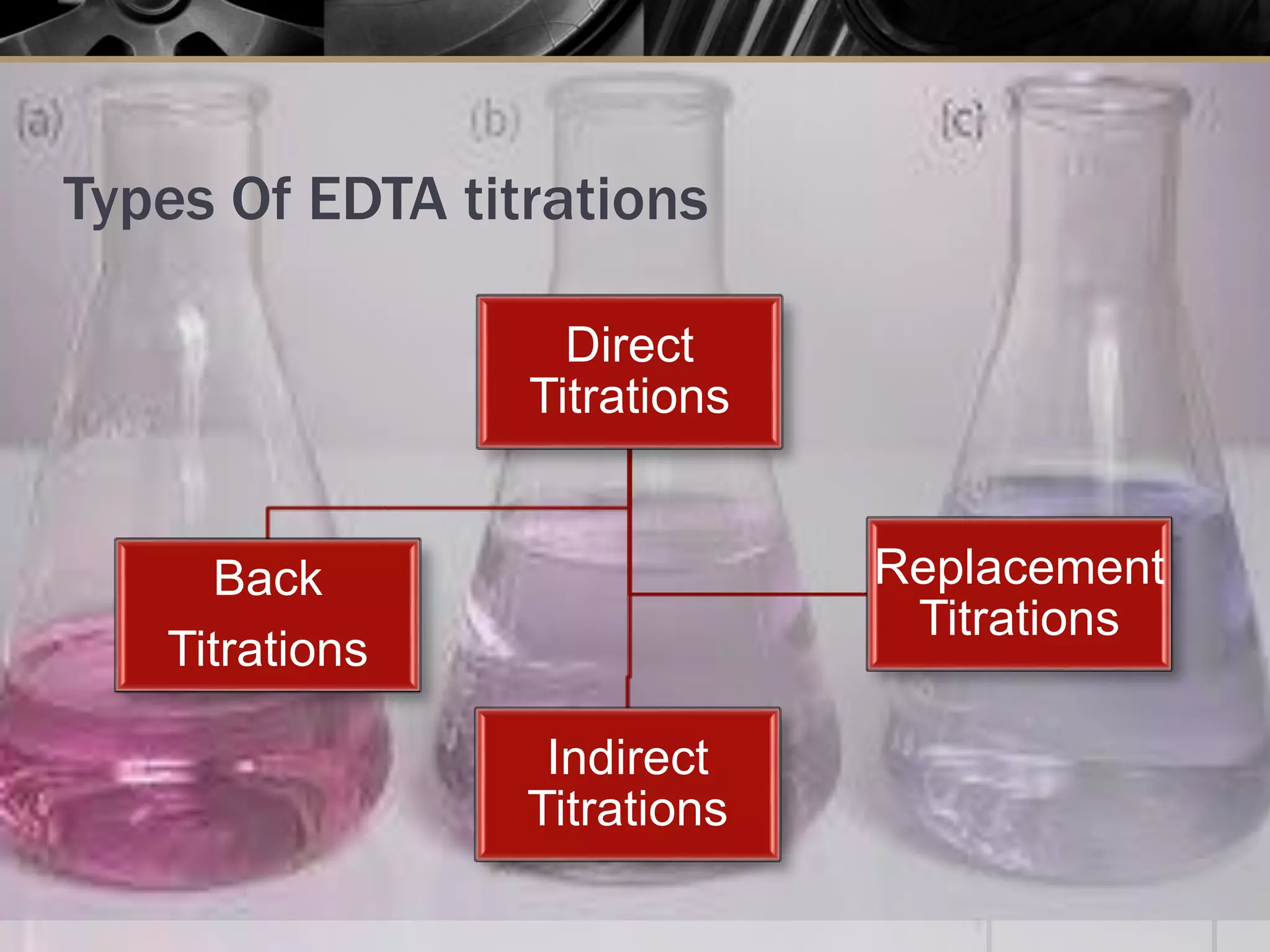
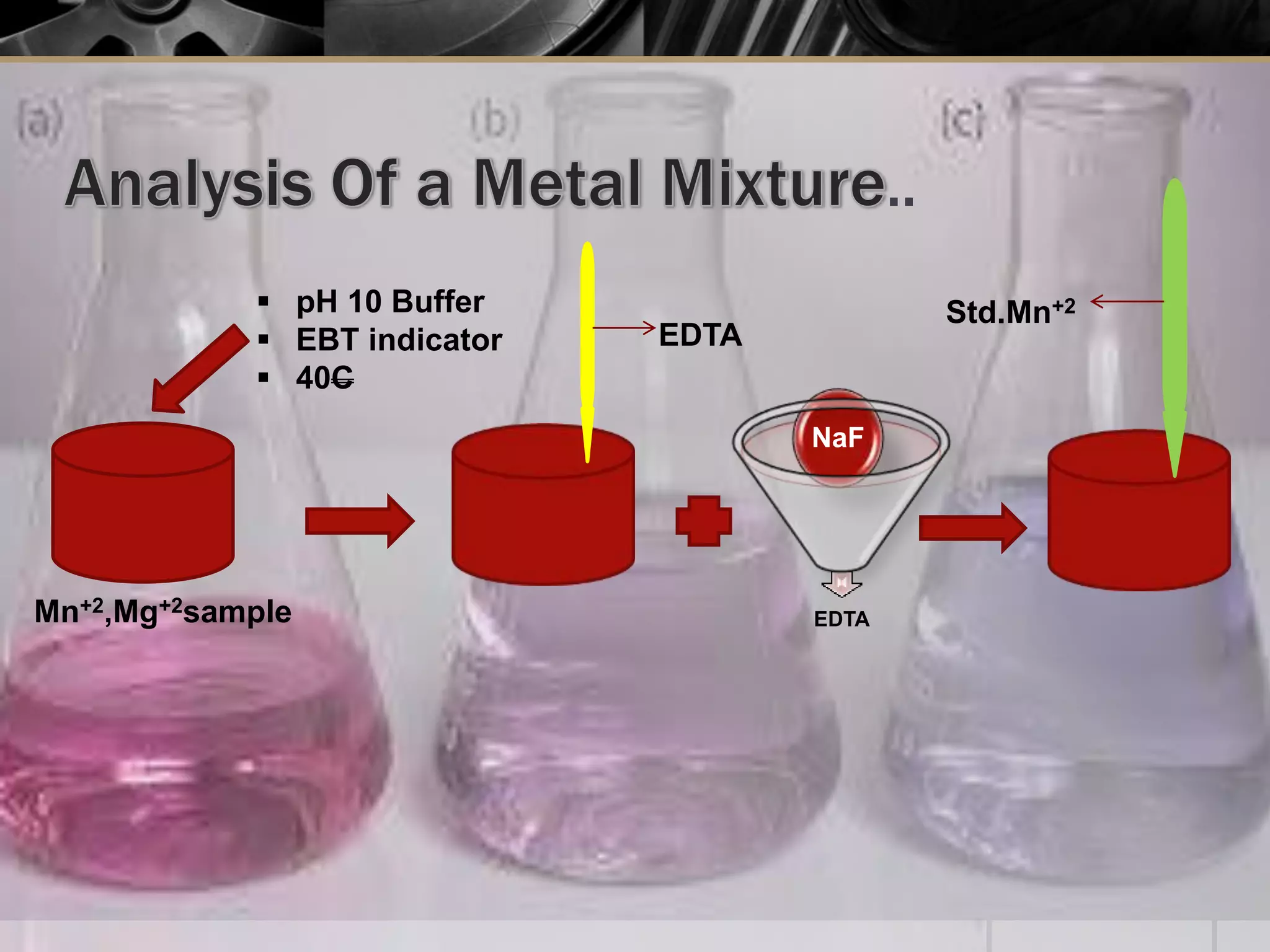
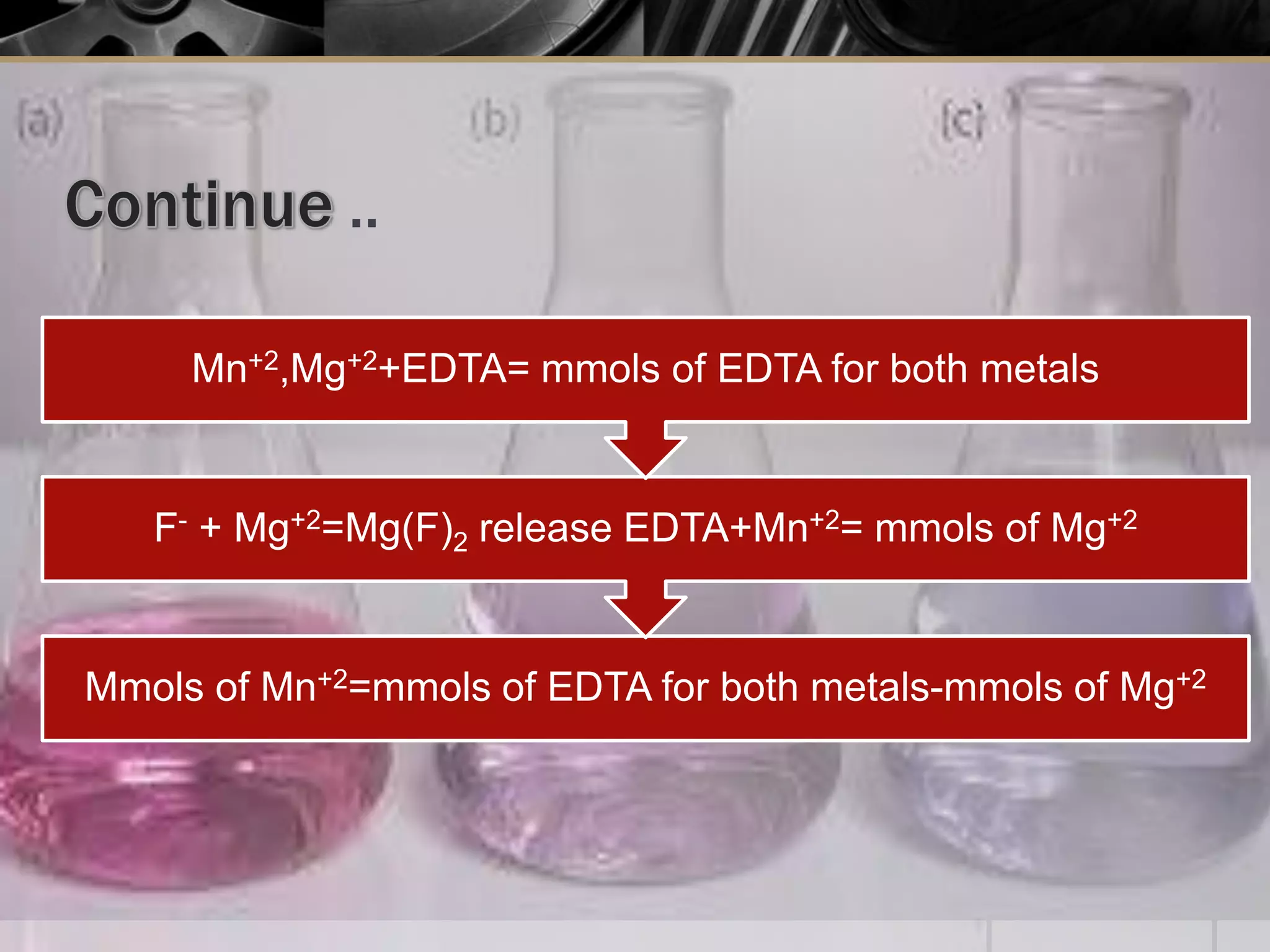
The document provides an overview of complexometric titration, detailing the use of EDTA as a chelating agent, types of titrations, and the importance of metallochromic indicators in visually detecting end points. It explains the mechanisms of EDTA titration with examples, including direct and back titrations, and discusses methods for enhancing selectivity through masking and demasking agents. Additionally, it outlines the stoichiometry, conditions for complex formation, and references for further reading.