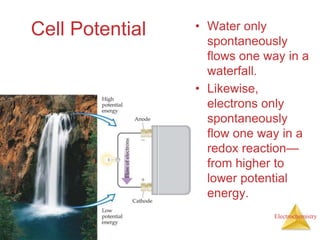
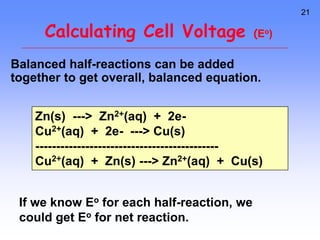
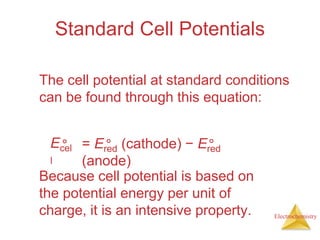
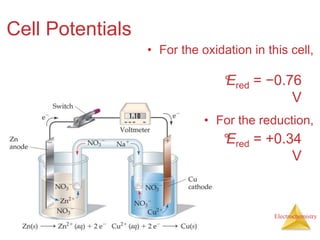
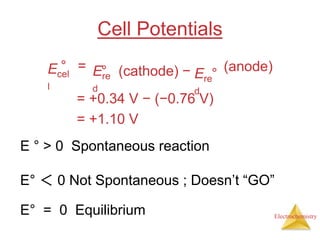
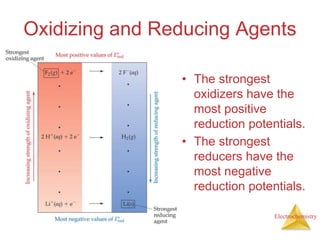
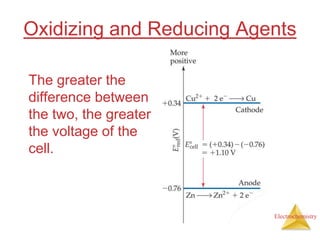
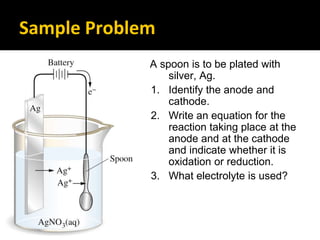
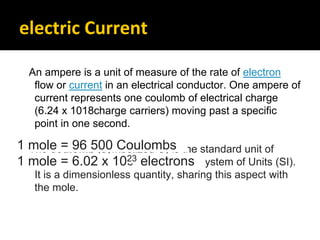
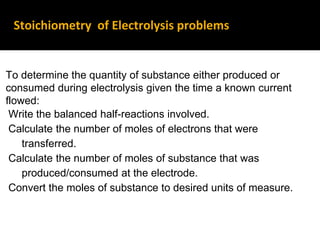
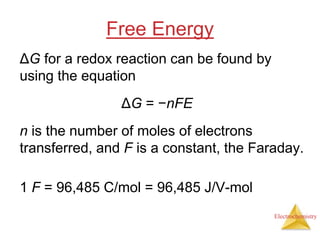
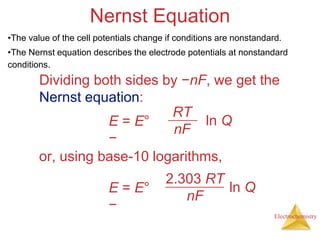
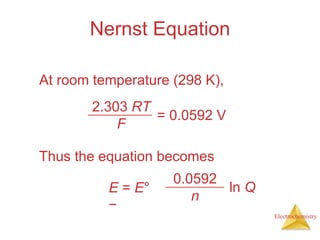
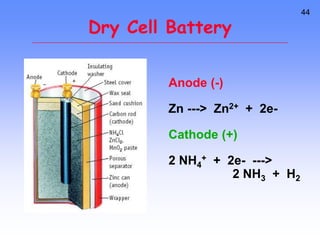
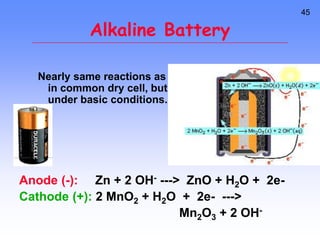
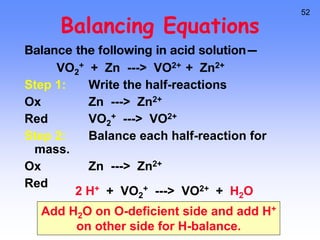
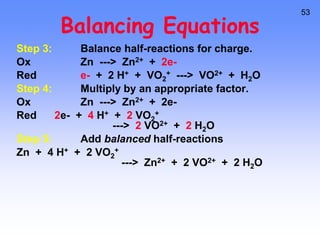
This document provides an overview of electrochemistry. It discusses electron transfer reactions, oxidation and reduction, voltaic cells, cell potentials, and balancing redox reactions. Key points include:
- Electron transfer reactions are oxidation-reduction or redox reactions that result in the generation of an electric current.
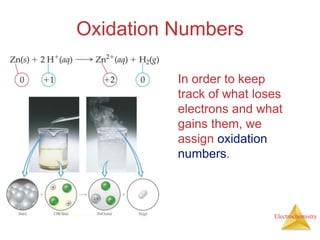
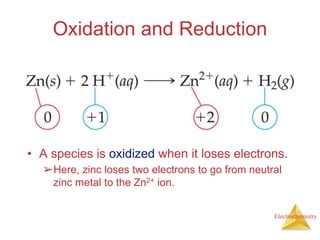
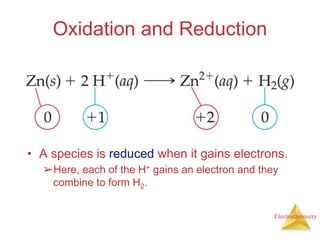
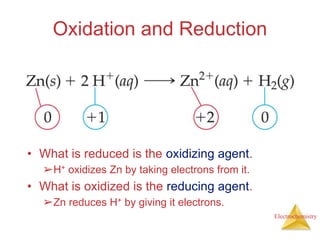
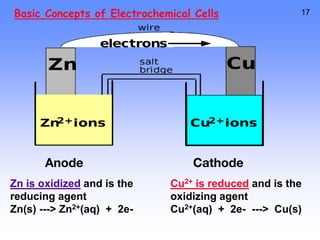
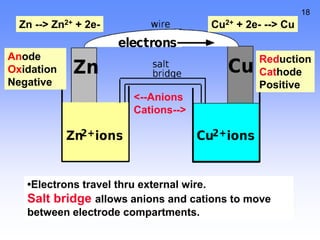
- Oxidation is the loss of electrons and reduction is the gain of electrons. Reduction cannot occur without oxidation providing electrons.
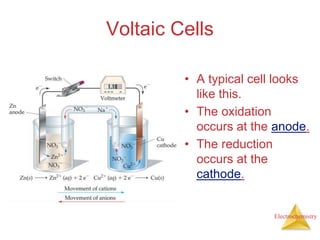
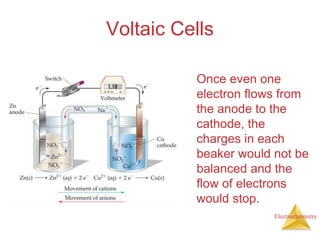
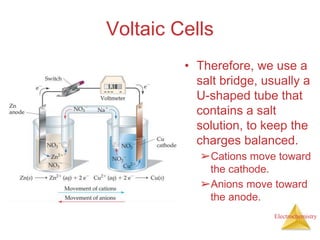
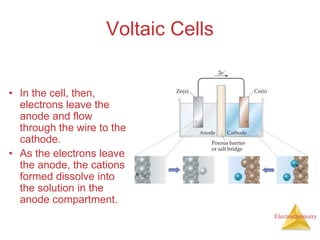
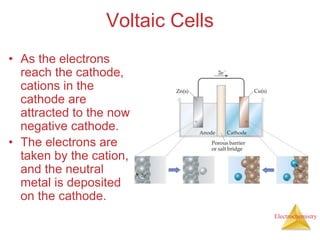
- A voltaic cell uses the energy from a spontaneous redox reaction to generate an electric current by transferring electrons through an external circuit between the anode and cathode.