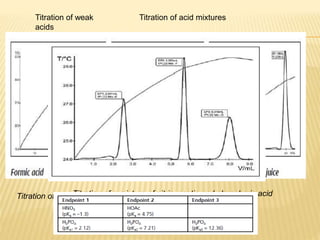
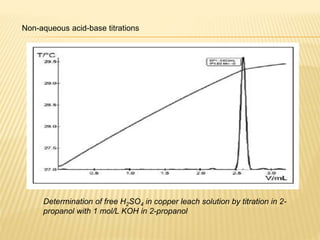
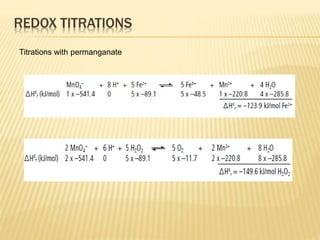
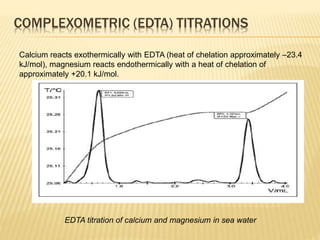
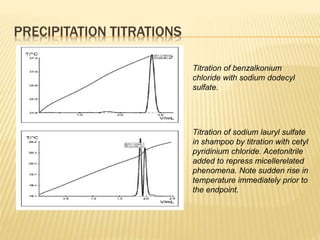
This document discusses thermometric titration, where the endpoint of a titration reaction is determined by measuring the temperature change. Key points:
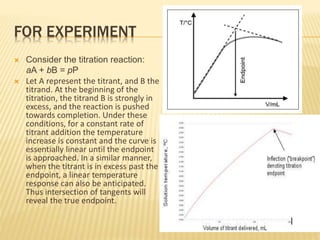
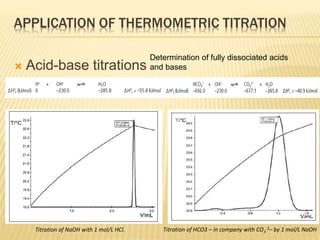
- Titrant is added continuously and the temperature change is measured, with the endpoint identified by an inflection point on the temperature curve.
- The temperature change observed is directly related to the enthalpy change of the reaction.
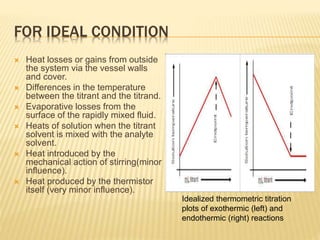
- Factors like heat losses, temperature differences between titrant and analyte, and stirring must be controlled for accurate results.
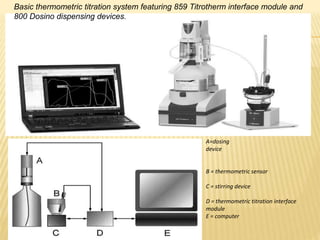
- Automated systems use burets for precise titrant addition, a thermistor probe for temperature measurement, and software for data collection and endpoint determination.
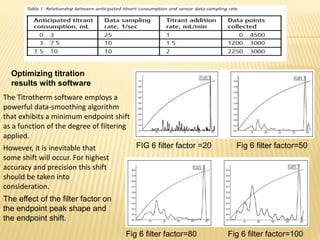
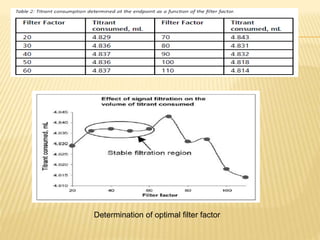
- Parameters like mixing, probe placement,