1. The document discusses different types of electrochemical cells including galvanic/voltaic cells and electrolytic cells.
2. Galvanic cells are further classified as primary cells, which cannot be recharged, and secondary cells, which are rechargeable batteries.
3. The Nernst equation is derived, which relates the electrode potential to the standard electrode potential and the concentrations of the metal ions involved in the electrochemical cell reaction.




![Oxidation: A species loses one or more electrons resulting in the increase in its
oxidation number.
Reduction: A species gain one or more electrons resulting in a decreasing in its
oxidation number.
Oxidation should accompanied by reduction, because if one losses electrons another
must ready to accept electrons. This reaction is called redox reaction.
Single electrode Potential:
It is defined as the potential developed at the interphase between the metal and the
solution, when a metal is dipped in a solution containing its own ions. It is
represented as E
Standard reduction potential (Eo) :
It is defined as potential developed at the interface between the metal and the
solution, when a metal is dipped in a solution containing its own ions of unit
concentration at 298K. [If the electrodes involve gases then it is one atmospheric
pressure] It is denoted as E0.
Electromotive force (EMF):
It is defined as the potential difference between the two electrodes of a galvanic cell
which causes the flow of current from an electrode with higher reduction potential to
the electrode with lower reduction potential.
It is denoted as E cell.
E cell = E right –E left.
E cell = E cathode – E anode.](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-5-320.jpg)


![Derivation of Nernst Equation for Electrode
potential
In 1889 Nernst derived a quantitative relationship between
the electrode potential and the concentrations of metal ions are
involved. The maximum work available from a reversible
chemical process is equal to the maximum amount of electrical
energy that can be obtained; it shows decrease in free energy.
Wmax = – ∆G--------[1]
And
Wmax = difference in potential between two electrode x
total quantity of charge flowing
through the cell
Total quantity of charge flowing through the cell = (No. of
moles of electrons) x (Faradays
constant)
So Wmax = nFEcell --------[2]](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-8-320.jpg)
![c
0
0
c
KlnGG
,isotherm'reactionhoffvant'abyrelatedareGandG,K
]n[M
[M]
c
K
aswrittenbecan
c
Kconstantequlibrium,reactionabovefor the
M-nenM
reaction,electrodereversibleaconsider
0nFE-0Gion,std.conditunder
[3]-----nFE-G
[2]&[1]eqnequate
RT
](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-9-320.jpg)
![]6[
]n[M
1
logRT0EE
1[M]condition,standardunder
]n[M
[M]
lnRT0EE
nF-bysidesboth thedivide
]n[M
[M]
ln
0
-nFEnFE-
equation,abovetheto
0
GandG,cKofvaluesthesubstitute
RT](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-10-320.jpg)
![Where,
E = Electrode potential
E0 = standard electrode potential
n = no. of electrons
[Mn+] = Concentration of metal ions
R = Universal gas constant = 8.314J K-1 mol-1
T = Temperature (In Kelvin) = 298K
]nlog[M
n
0.05910EE
eqn[6],thetovaluesthesubstitute
](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-11-320.jpg)




![
eCd
M
Cd 2
2
)1(
)2(
2
2
M
CdeCd
Electrode concentration cells
In these cells, the potential difference is developed between two electrodes
at different concentrations dipped in the same solution of the electrolyte.
For example,
Cd-Hg (M1) | CdSO4 | Cd-Hg (M2)
Two Cd-Hg electrodes of different concentration immersed in a CdSO4 solution.
Electrode containing high concentration of metal acts as a anode , where oxidation
occurs
Electrode containing low concentration of metal acts as a cathode, where reduction
occurs
M2.M1only whenveisEcell
]2[
]1[
log
2
0591.0
]1log[
2
0591.00]2log[
2
0591.00
bewillcellionconcentrattheofemfThe
M
M
cell
E
MEME
cell
E
anodecathodecell EEE](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-16-320.jpg)



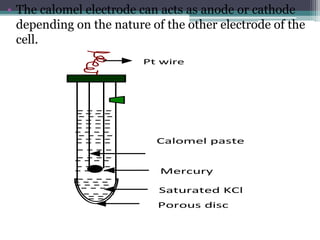

![Concentration
of KCl
E0
[v]
saturated KCl 0.241
1M KCl 0.281
0.1M KCl 0.33
4
MEASUREMENT OF ELECTRODE POTENTIAL USING CALOMEL ELECTRODE:
Electrode potential of a given electrode can be measured by using calomel electrode as a
reference electrode.
Example: To measure the electrode potential of zinc, Zinc electrode is coupled with SCE. So zinc
acts as anode and SCE acts as cathode
cellZnZn
cellcathodeanode
anodecathodecell
EE
EEE
EEE
241.02
/
Applications:
1. It is used as secondary reference electrode in the measurement of single electrode.
2. It is used as reference electrode in all potentiometer determinations and
to measure pH of the given solution](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-22-320.jpg)
![Construction and working of Silver- Silver Chloride
electrode:
• Silver-Silver chloride is also a metal-metal salt ion electrode.
• Silver and its sparingly soluble salt silver chlorides are in
contact with a solution of chloride ions. Generally a silver wire
is coated with AgCl and dipped in a solution of KCl .
• Cell representation is as follows
Ag |AgCl | Cl-
Concentration of KCl E0 [v]
saturated KCl 0.241
1M KCl 0.281
0.1M KCl 0.334](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-23-320.jpg)



![WORKING:
• The glass electrode works on the principle that when a thin
glass membrane is placed between two different concentration
of a solution, a boundary potential Eb is developed at layers of
the glass membrane. This potential arises due to difference in
the concentration of H+ ion inside and outside the membrane.
External Solution glass membrane Internal solution
C2=[H+] C1=[CONSTANT]=k
E2 Eb E1
• Boundary potential, Eb = E2 – E1](https://image.slidesharecdn.com/emf-160704175829/85/MODULE-1-ELECTROCHEMISTRY-27-320.jpg)







