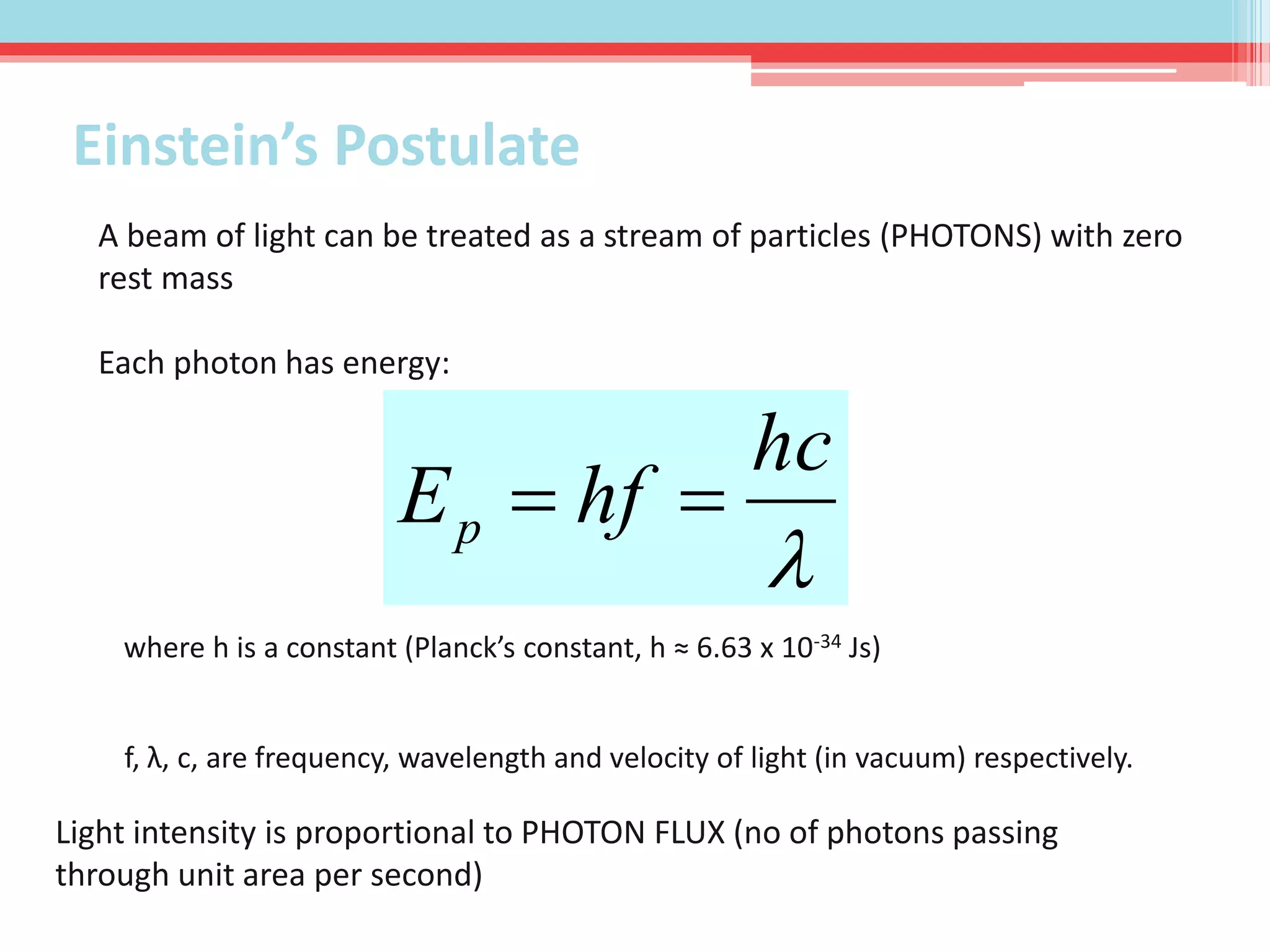
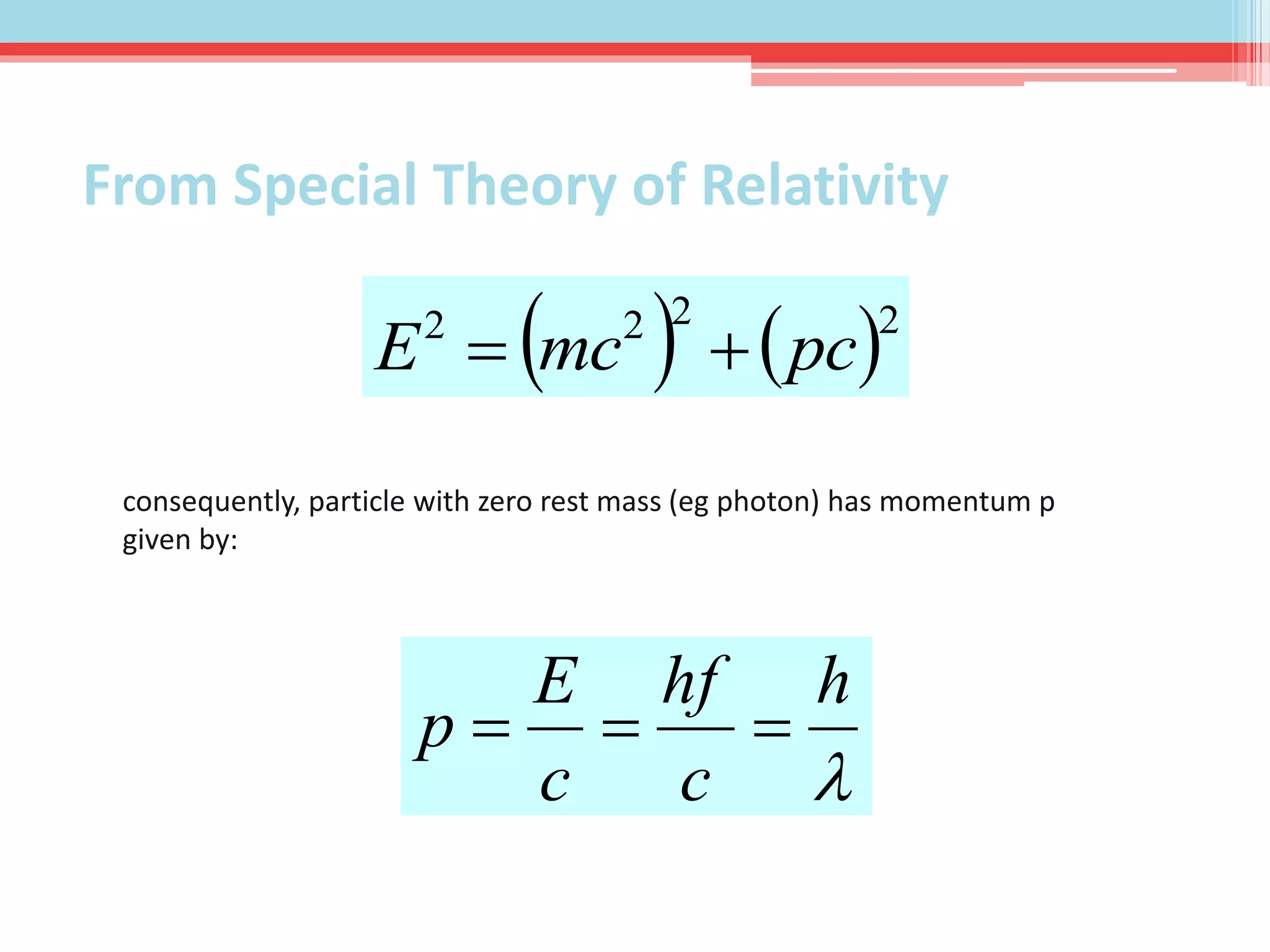
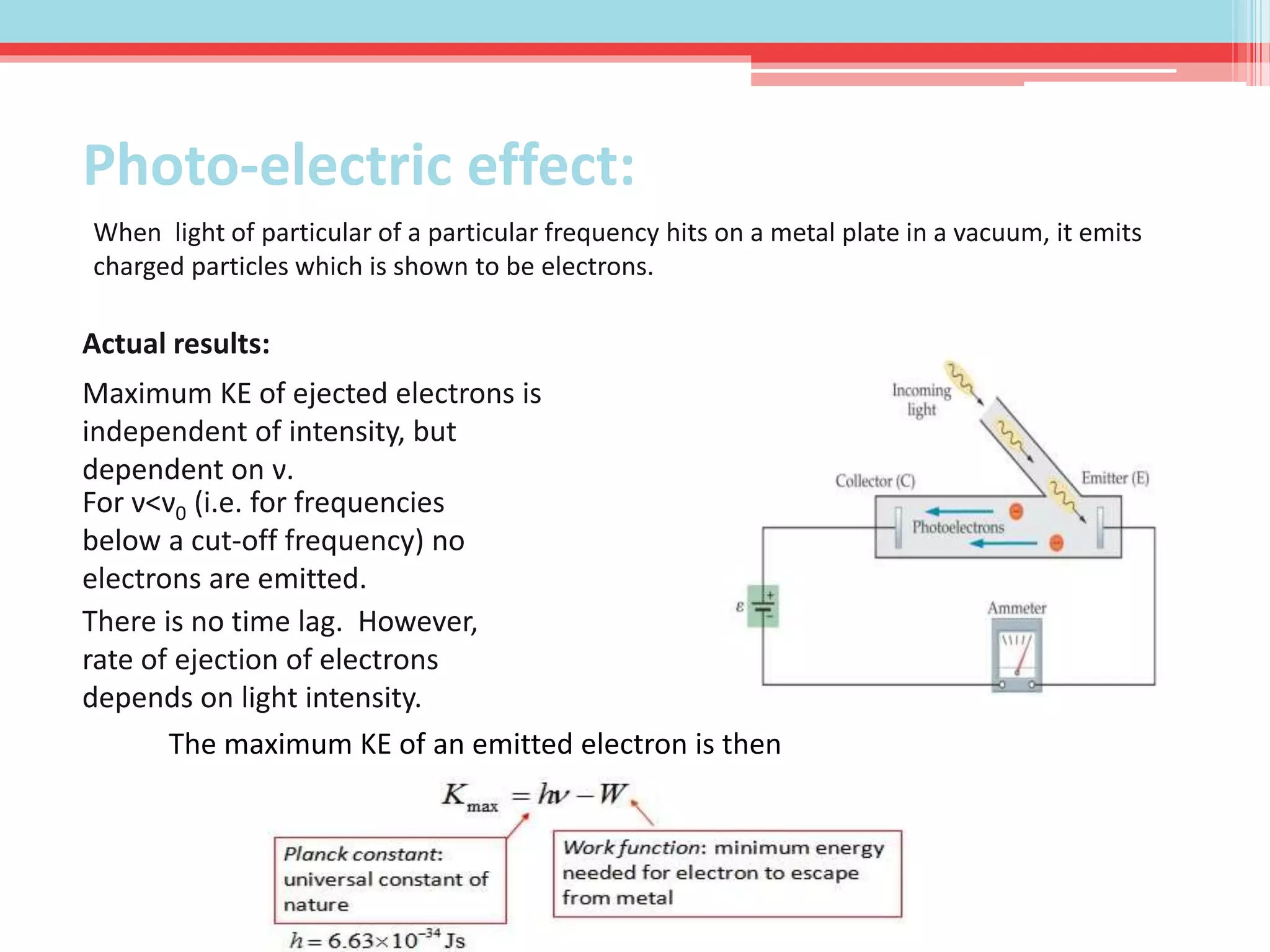
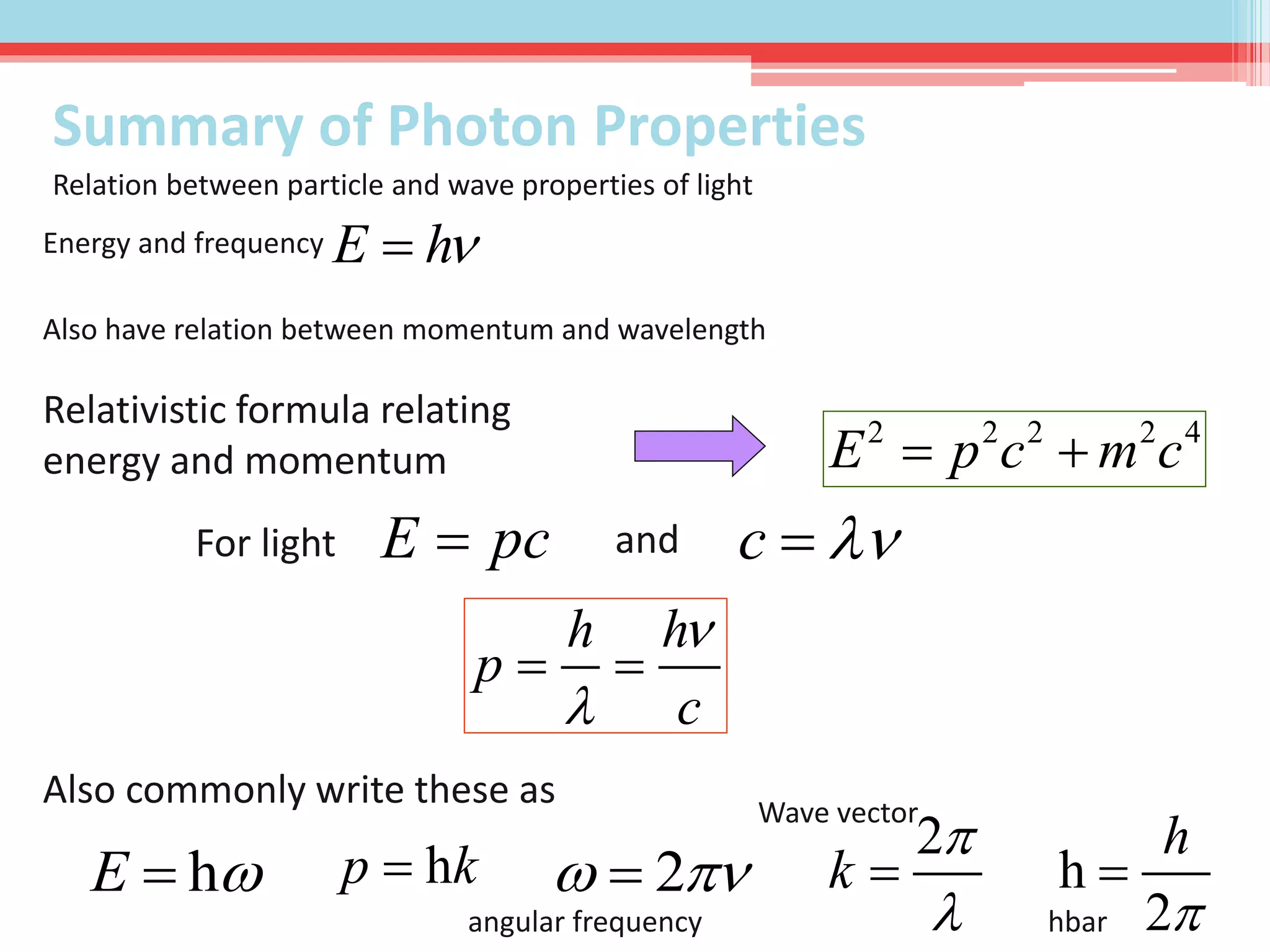
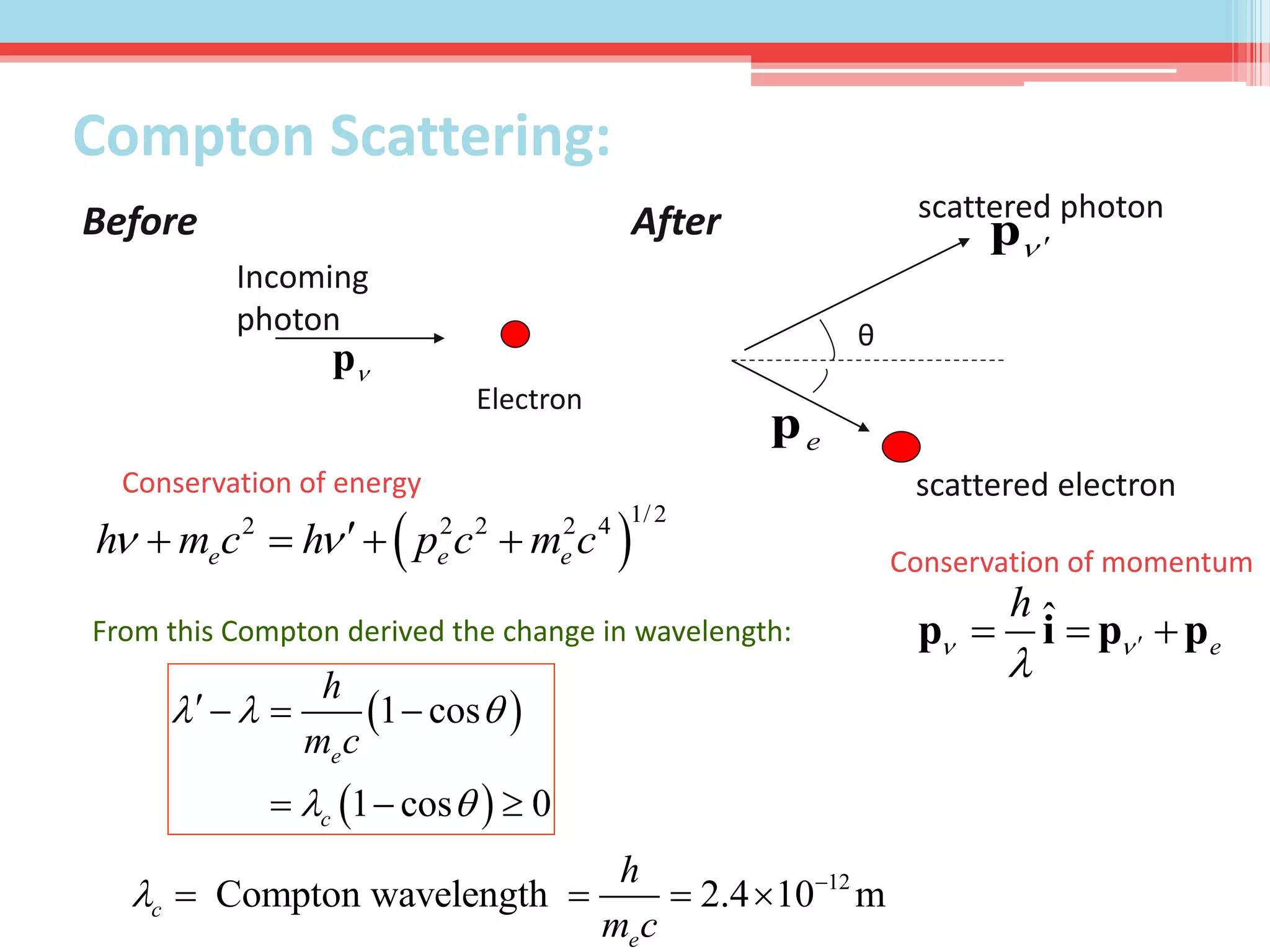
This document summarizes Louis de Broglie's hypothesis of wave-particle duality and its applications. It discusses de Broglie's proposal that particles have wave-like properties with a wavelength given by Planck's constant divided by momentum. The photoelectric effect and Compton effect provide evidence of wave and particle behavior of light and electrons. Wave-particle duality is exploited in technologies like electron microscopy and neutron diffraction to examine structures smaller than visible light wavelengths. While useful, wave-particle duality does not fully explain quantum phenomena like the Heisenberg uncertainty principle.