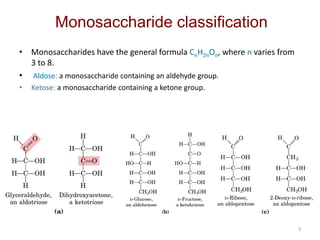
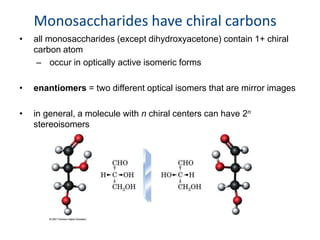
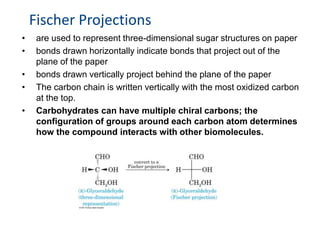
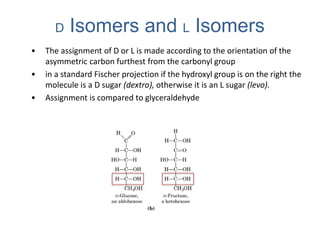
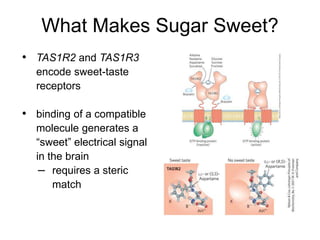
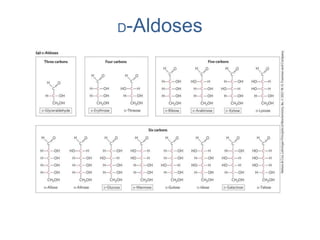
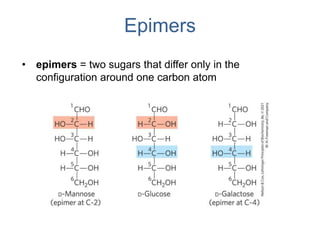
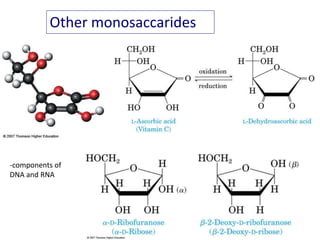
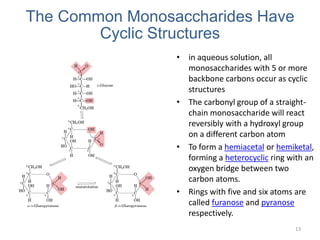
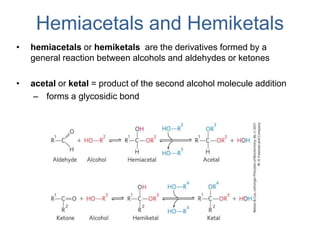
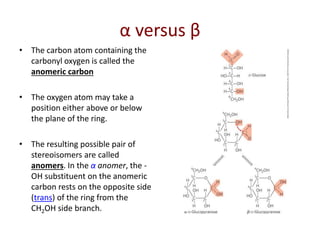
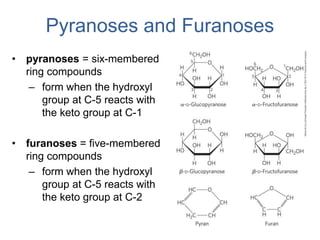
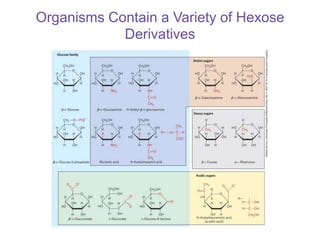
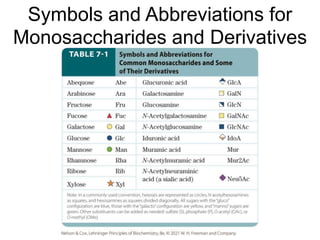
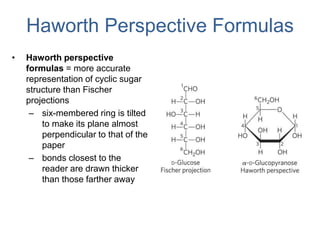
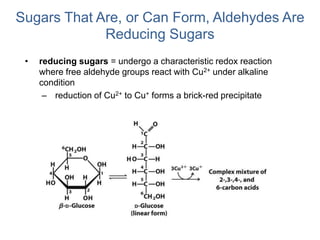
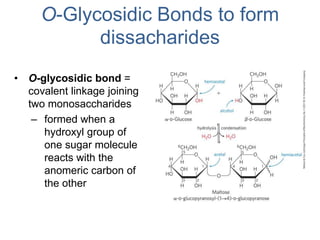
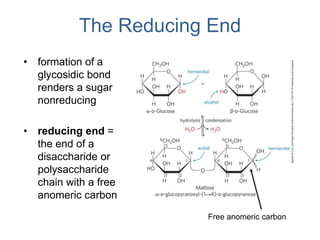
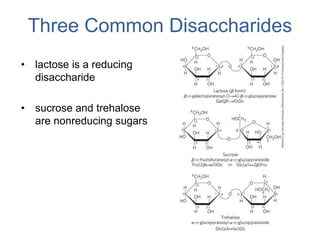
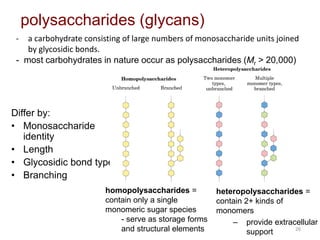
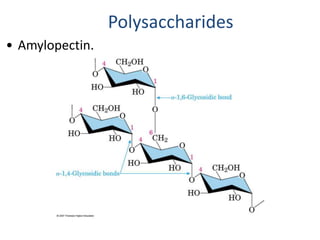
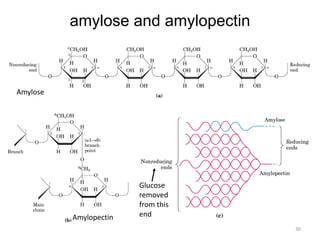
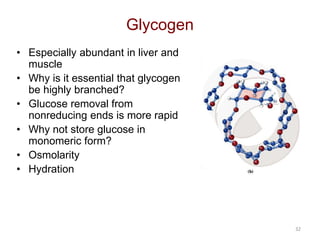
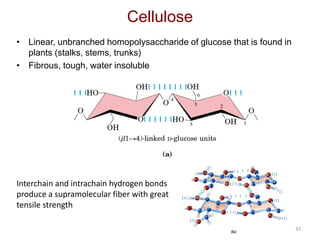
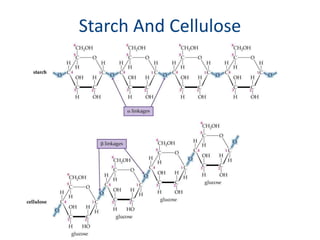
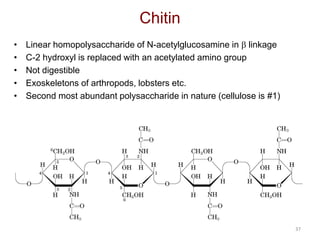
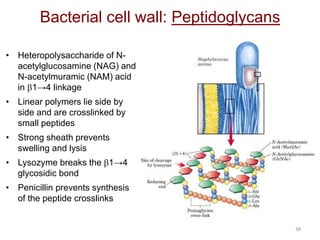
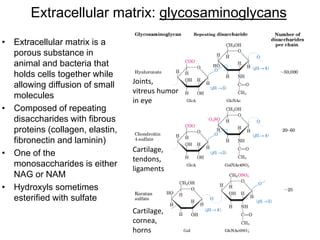
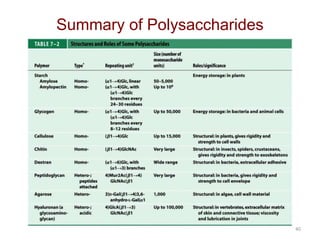
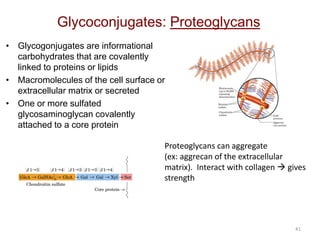
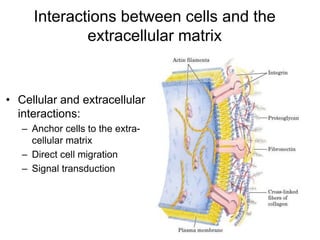
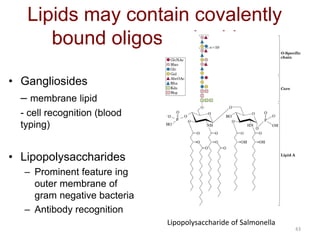
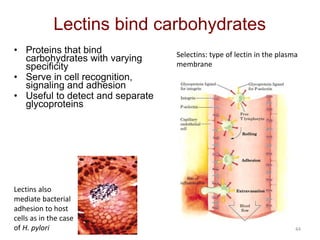
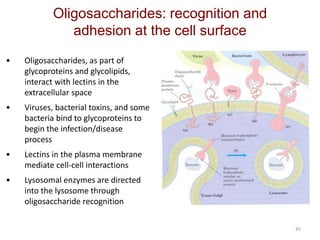
Carbohydrates are made up of carbon, hydrogen, and oxygen. They exist as monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides can be aldoses or ketoses and exist as cyclic structures in solution. Disaccharides like sucrose are formed through glycosidic bond formation between two monosaccharides. Polysaccharides vary in composition and function, with starch and glycogen serving as energy storage and cellulose providing structural support. Glycoconjugates are carbohydrates bound to proteins or lipids that play important roles in cell signaling and recognition.