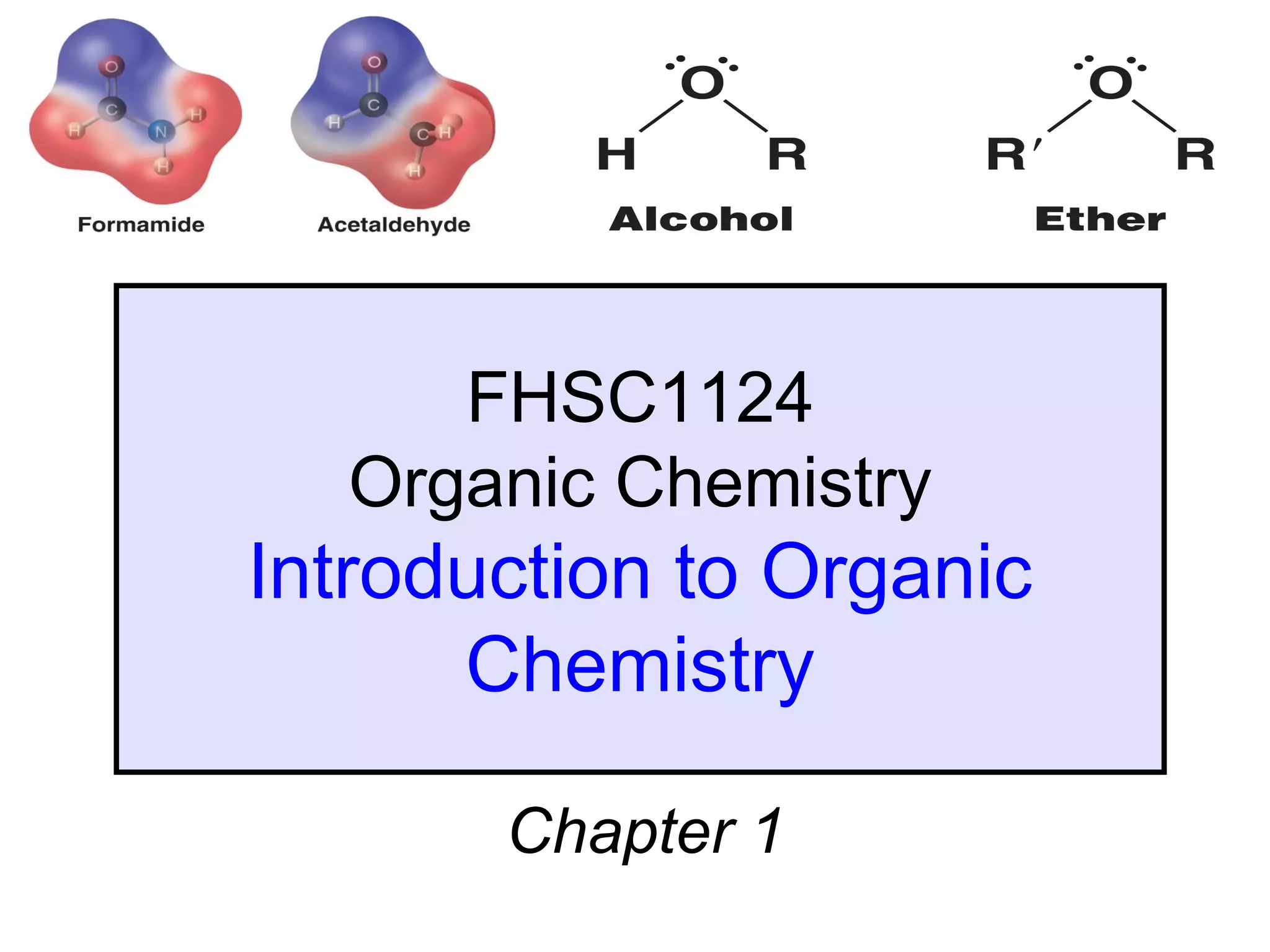
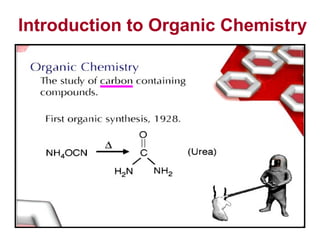
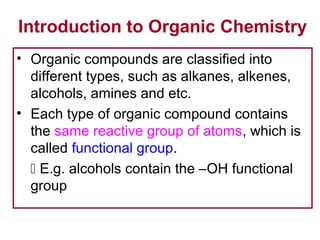
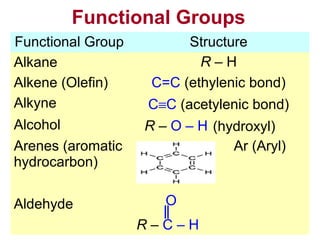
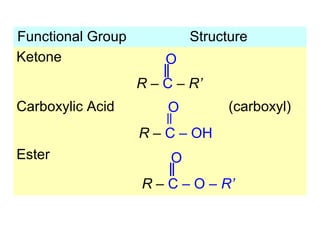
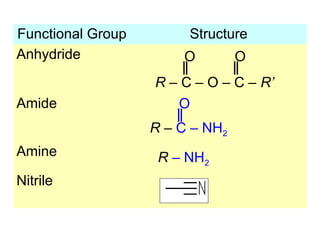
1. The chapter introduces organic chemistry and the different functional groups that classify organic compounds.
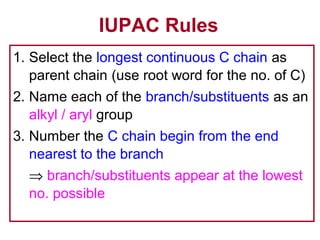
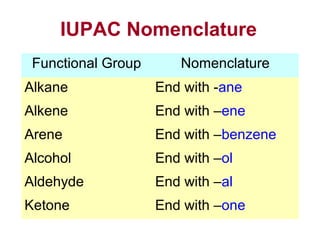
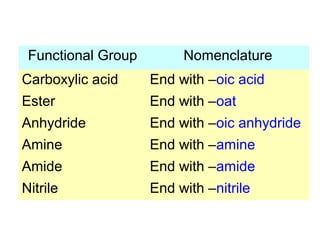
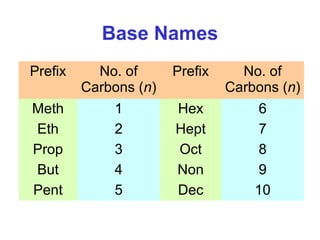
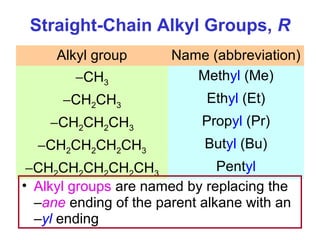
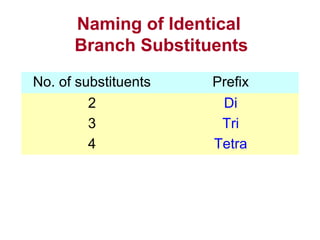
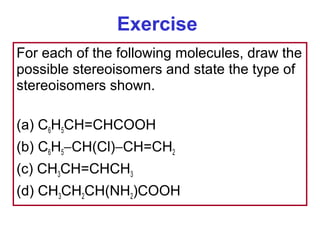
2. It describes IUPAC nomenclature rules for systematically naming organic structures and explains how to identify substituents.
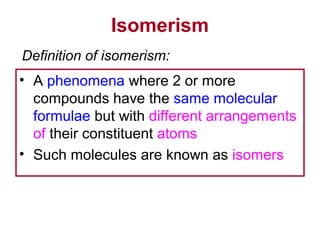
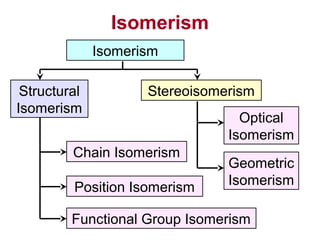
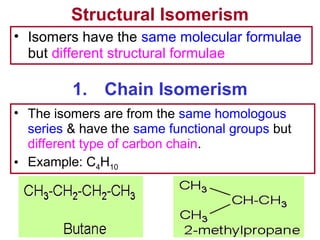
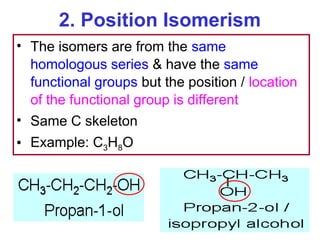
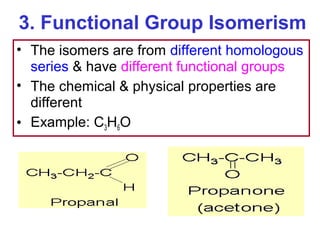
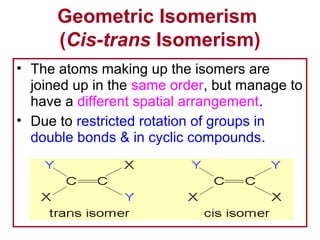
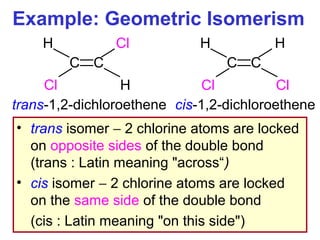
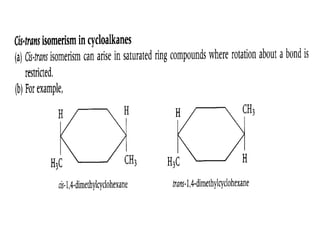
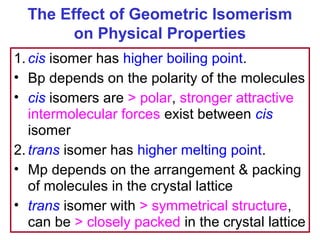
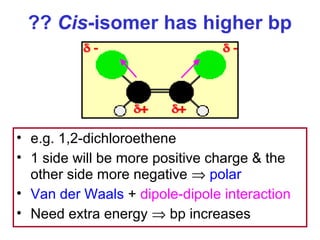
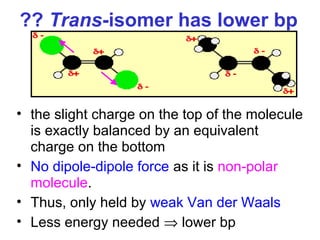
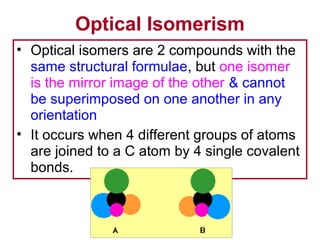
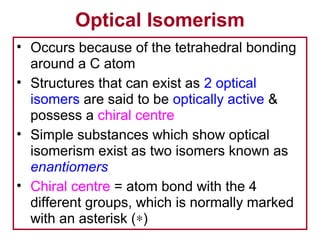
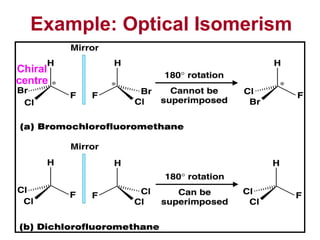
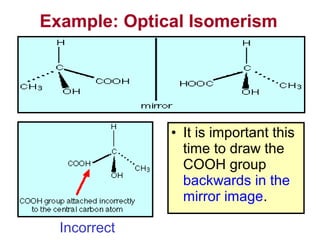
3. The chapter covers different types of isomerism including structural, stereoisomers, and optical isomers that can exist.