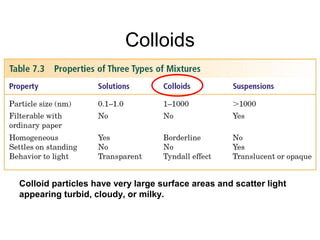
This document provides an overview of solutions and colloids. It defines key terms like solvent, solute, solubility, saturated and unsaturated solutions. Factors that affect solubility include nature of solute and solvent, temperature, and pressure. Concentrations of solutions can be expressed as molarity, percent composition, or parts per million. Colligative properties depend only on the number of solute particles and include lowering of vapor pressure, freezing point, and boiling point. Osmosis is the movement of solvent through a semipermeable membrane, and osmotic pressure is the pressure needed to prevent osmosis. Dialysis uses semipermeable membranes to remove small solute molecules from the