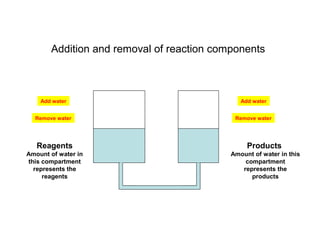
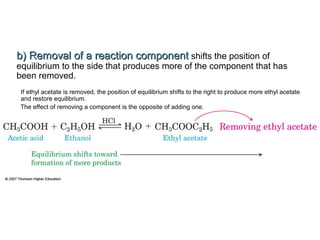
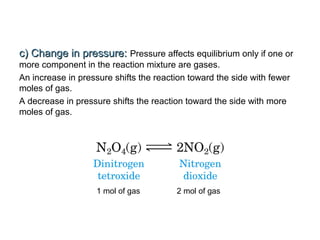
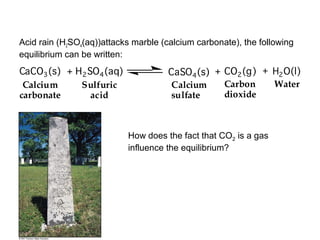
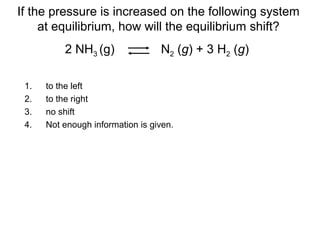
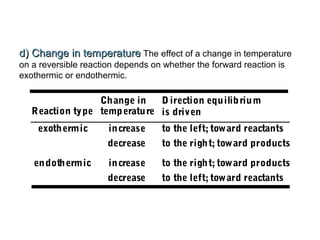
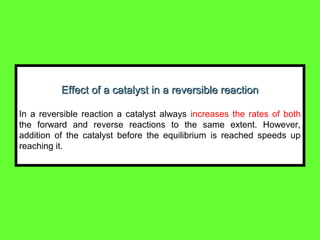
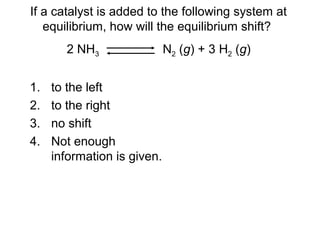
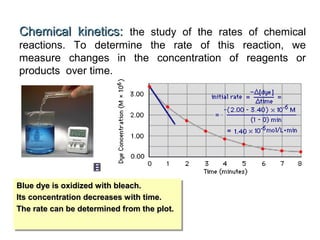
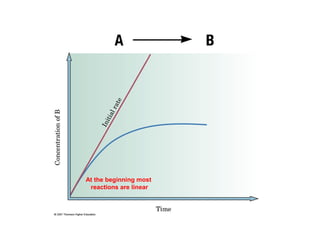
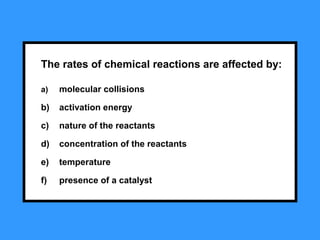
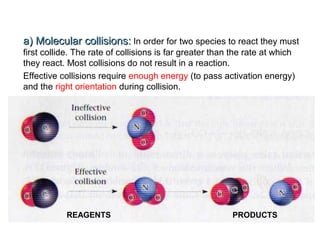
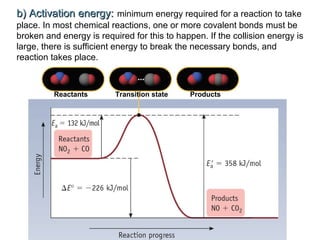
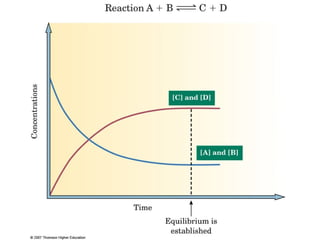
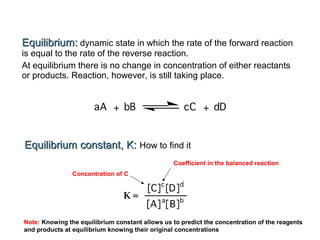
The document summarizes key concepts relating to chemical kinetics and chemical equilibrium. It discusses how the rates of chemical reactions are determined by measuring changes in concentration over time. It also explains how reaction rates are affected by molecular collisions, activation energy, nature of reactants, concentration, temperature, and presence of catalysts. The document introduces chemical equilibrium as a dynamic steady state and defines equilibrium constants. It describes Le Chatelier's principle, explaining how changing concentrations, temperature, or pressure shifts equilibrium.




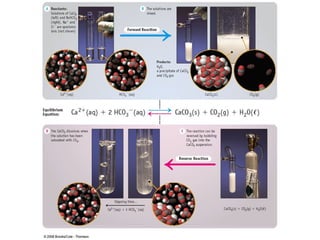
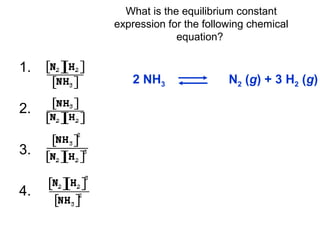
![Write the equilibrium constant expression for this reversible reaction:
Note: By convention the exponent “1” is understood but not written. K has no units because molarities
cancel.
When H2 and I2 react at 427°C, the following equilibrium is reached:
The equilibrium concentrations are [I2] = 0.42 mol/L, [H2] = 0.025 mol/L,
and [HI] = 0.76 mol/L. Using these values, calculate the value of K.](https://image.slidesharecdn.com/chapter7-171030220655/85/Chapter-7-21-320.jpg)