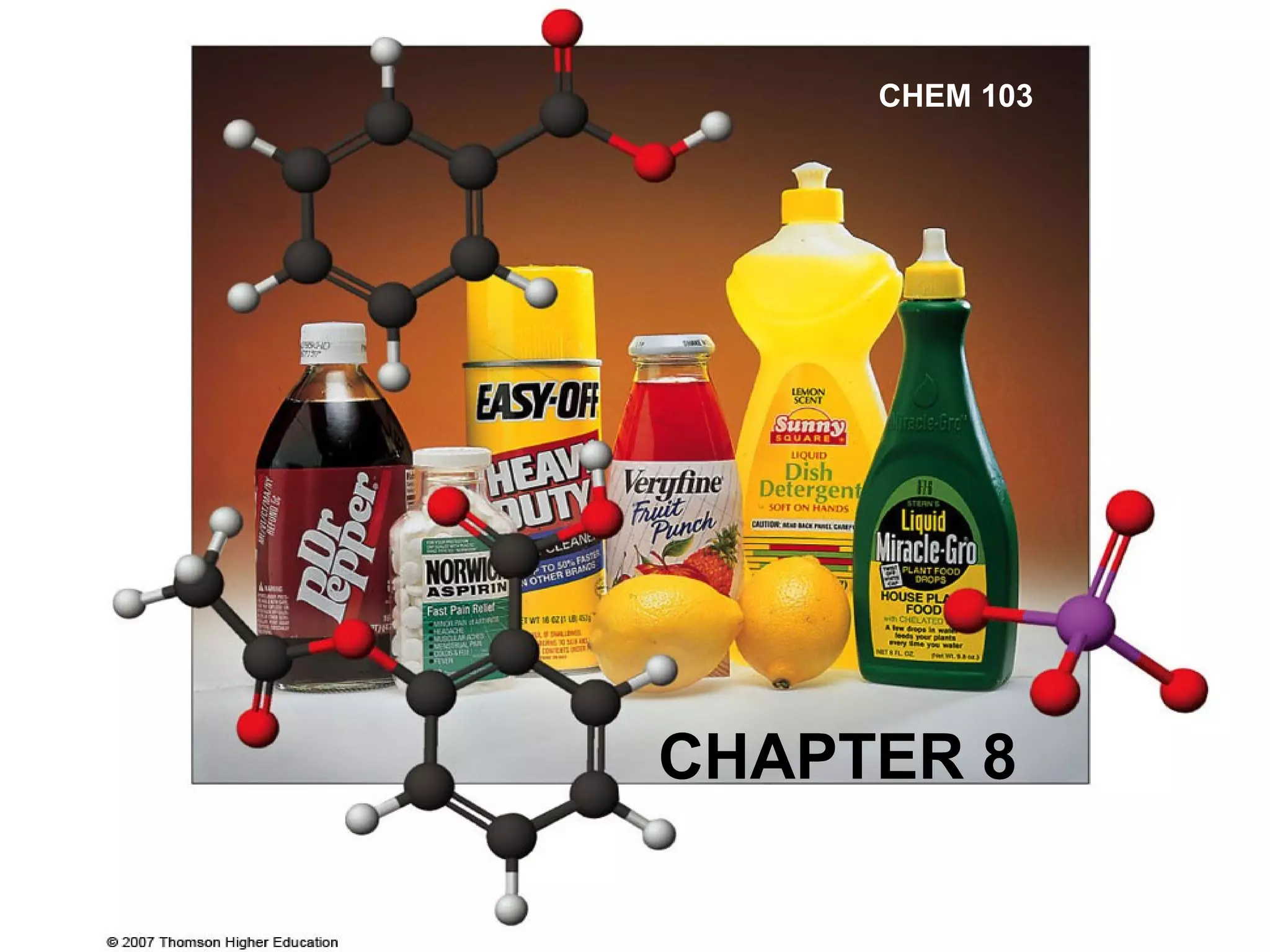
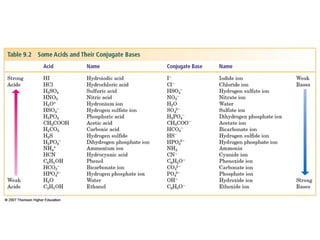
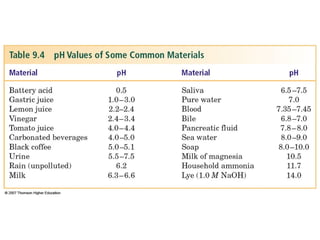
This document summarizes key concepts about acids and bases from a chemistry textbook chapter:
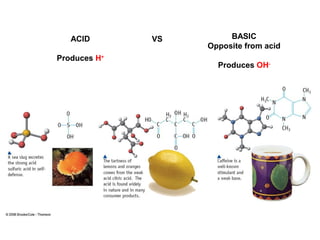
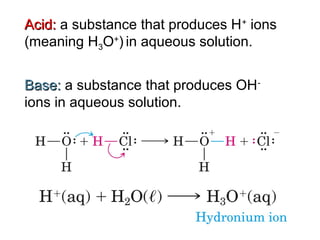
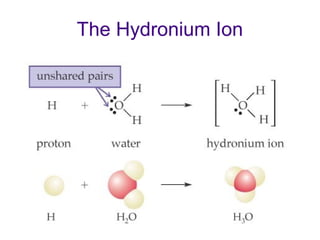
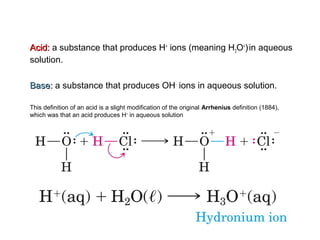
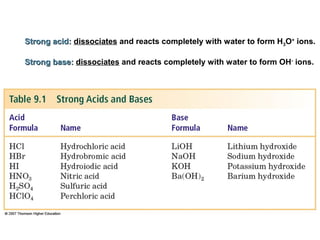
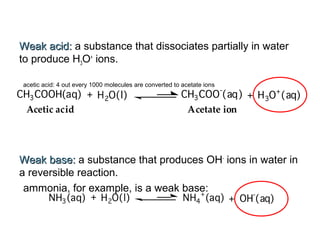
1. It defines acids as substances that produce H+ ions in aqueous solution and bases as substances that produce OH- ions. Strong acids and bases dissociate completely while weak acids and bases dissociate partially.
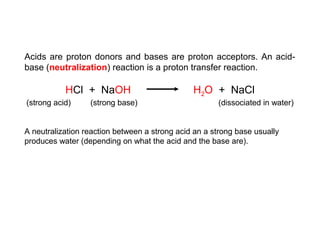
2. The pH scale is introduced and used to classify solutions as acidic, basic or neutral. Common acid-base reactions like neutralization and self-ionization of water are described.
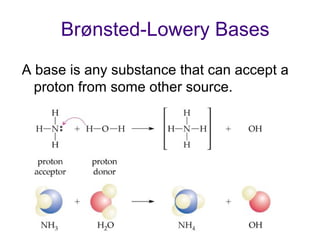
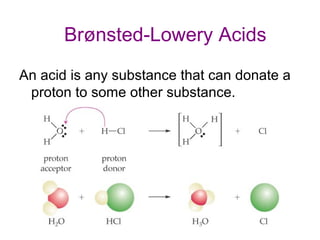
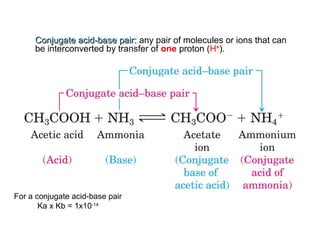
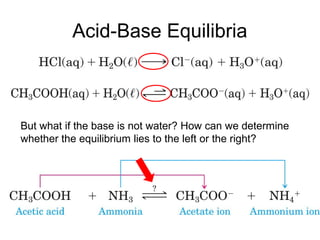
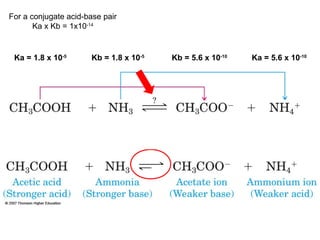
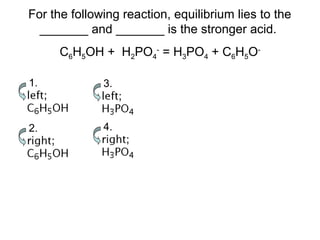
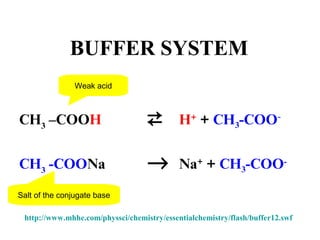
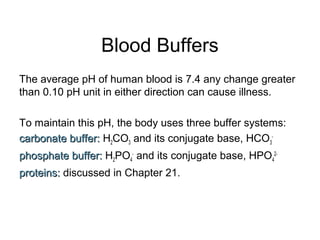
3. Key acid-base concepts are explained like Brønsted-Lowry acids and bases, conjugate acid-base pairs, acid-base equilibria, and the importance of pH buffers in biological systems like



![Many bases like KOH, NaOH, Mg(OH)2, and Ca(OH)2] disassociate in
water producing OH-
ions (basic solution):
Other bases produce OH-
by reacting with water molecules, here shown
for ammonia:](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-12-320.jpg)


![Equilibrium/Ionization Constants
When a weak acid, HA, dissolves in water
the equilibrium constant expression, Keq, for this ionization is:
Water is the solvent and its concentration changes very little when we add HA,
we treat [H2O] as a constant equal to 1000 g/L or 55.5 mol/L. We combine the
two constants to give the acid ionization constant, Ka:](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-19-320.jpg)



![Self-Ionization of Water
14-
3
2
2
10x1.0
]][[][
=
== −+
w
w
K
OHOHOHKK
Compare to [H2O] = 55.5 mol/L
-log[1.0 x 10-7
] = 7= pH water](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-31-320.jpg)
![The product of [H3O+
] and [OH-
] in any aqueous solution is
equal to 1.0 x 10-14
.
If we add 0.010 mol of HCl to 1.00 liter of pure water, in
this solution [H3O+
] = 0.010 or 1.0 x 10-2
. This means that
the concentration of hydroxide ion is:
wKOHOH ==−+ -14
3 10x1.0]][[](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-32-320.jpg)
![pH and pOH
Because hydronium ion concentrations for most solutions
are numbers with negative exponents, we express these
concentrations as pH:
pH = -log [H3O+
]
Acidic solution:Acidic solution: pH < 7.0.
Basic solution:Basic solution: pH > 7.0.
Neutral solution:Neutral solution: pH = 7.0.](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-33-320.jpg)
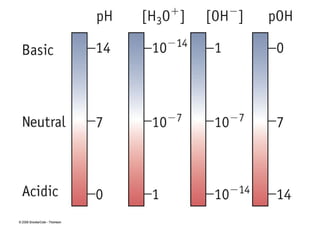
![if we know the pH of an aqueous solution, we can easily
calculate its pOH:
pOH = -log[OH-
]
the ion product of water, Kw, is 1.0 x 10-14
taking the logarithm of this equation gives:
pH + pOH = 14](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-35-320.jpg)
![1. acidic.
2. basic.
3. neutral.
4. radioactive.
If a solution has a [OH-
] of 4.87 x 10-9
M, then the
solution is classified as:](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-37-320.jpg)

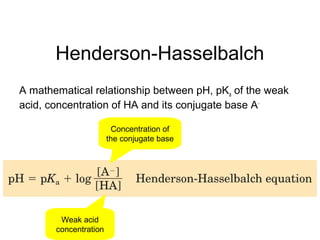
![pH = pKa
+ log
[CO3
2-
]
= - log (4.8e-11) + log
0.310
= 10.16
[HCO3
-
] 0.443
Substituting these values in the equation gives:
A buffer solution is 0.443 M in KHCO3 and 0.310 M in K2CO3.
If Ka for HCO3
-
is 4.8e-11
, what is the pH of this buffer
solution?](https://image.slidesharecdn.com/chapter8-171106043205/85/Chapter-8-43-320.jpg)