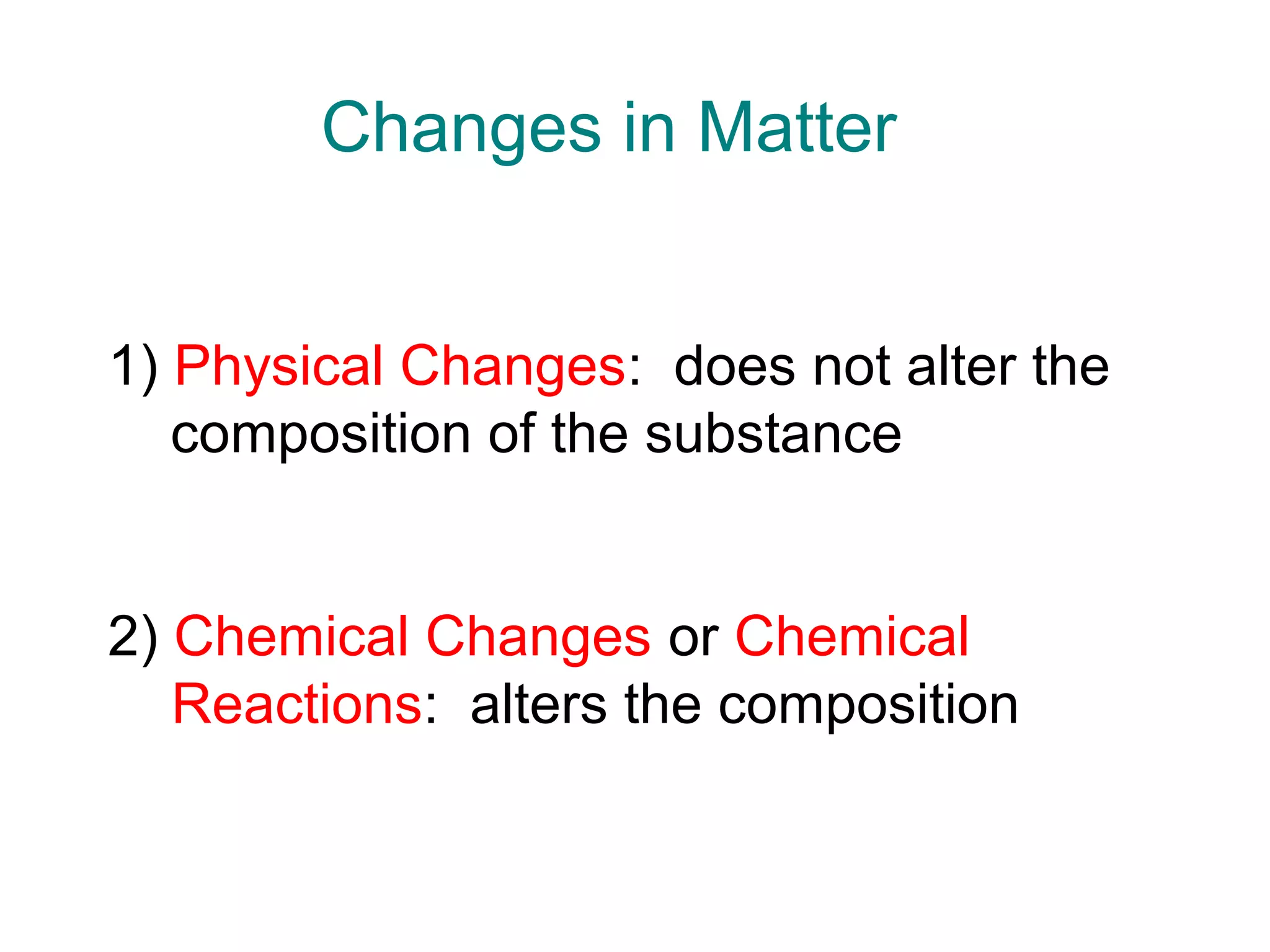
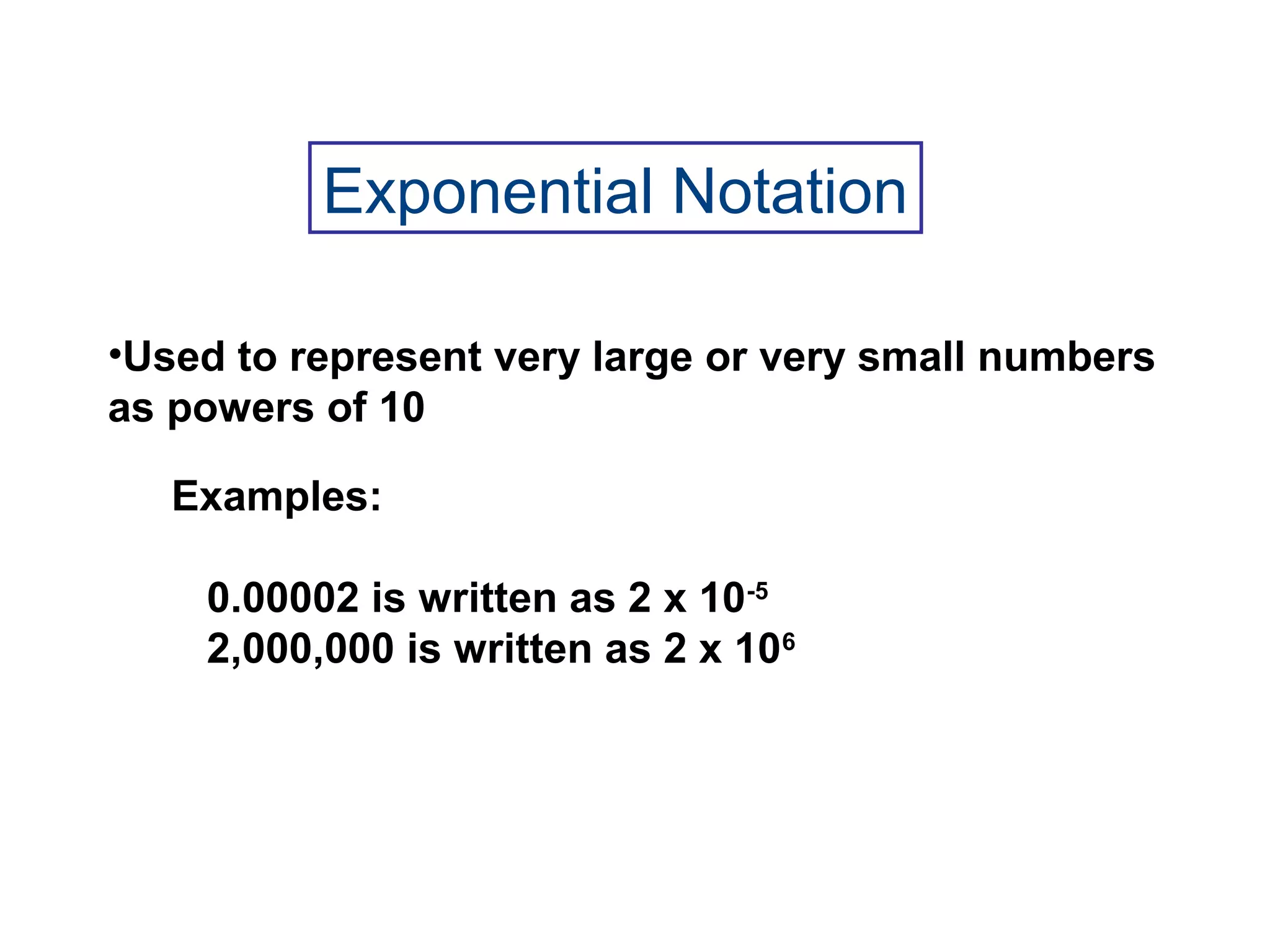
1) Chemistry is the study of matter and its properties. Matter can exist as solids, liquids, or gases and can undergo physical or chemical changes.
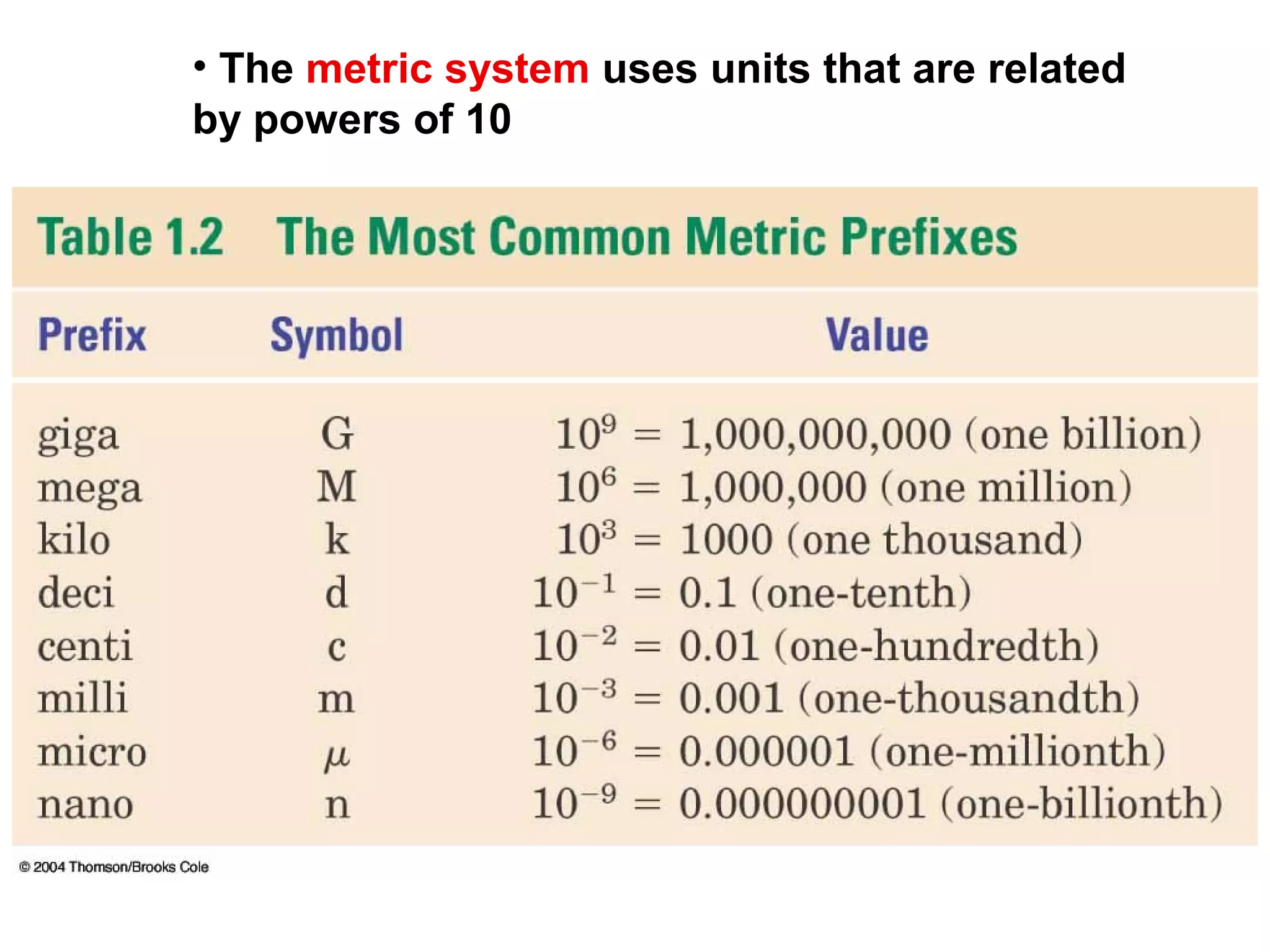
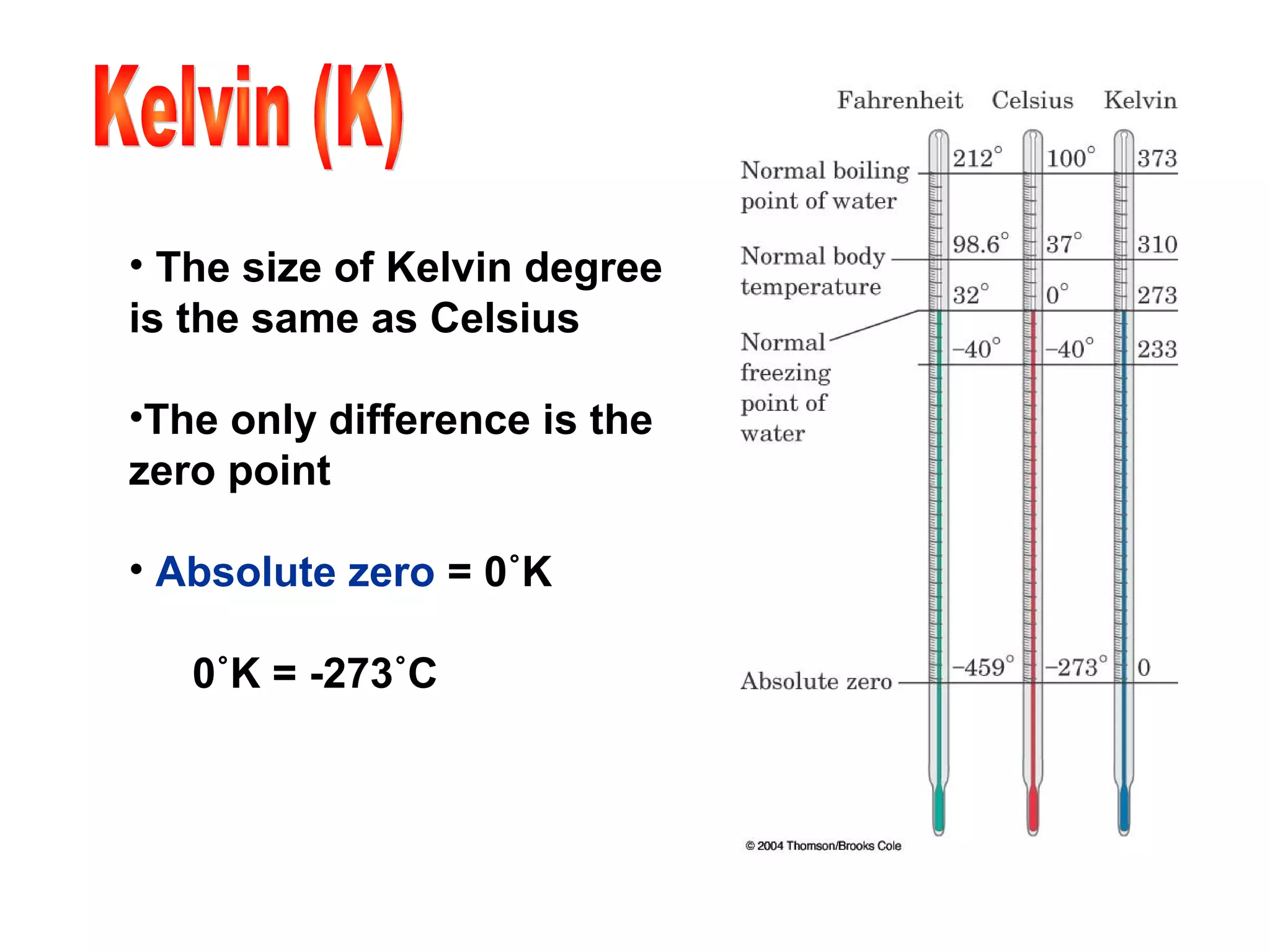
2) The metric system is the international standard for measurement and uses base units related by powers of 10. Measurements include mass, volume, length, and temperature.
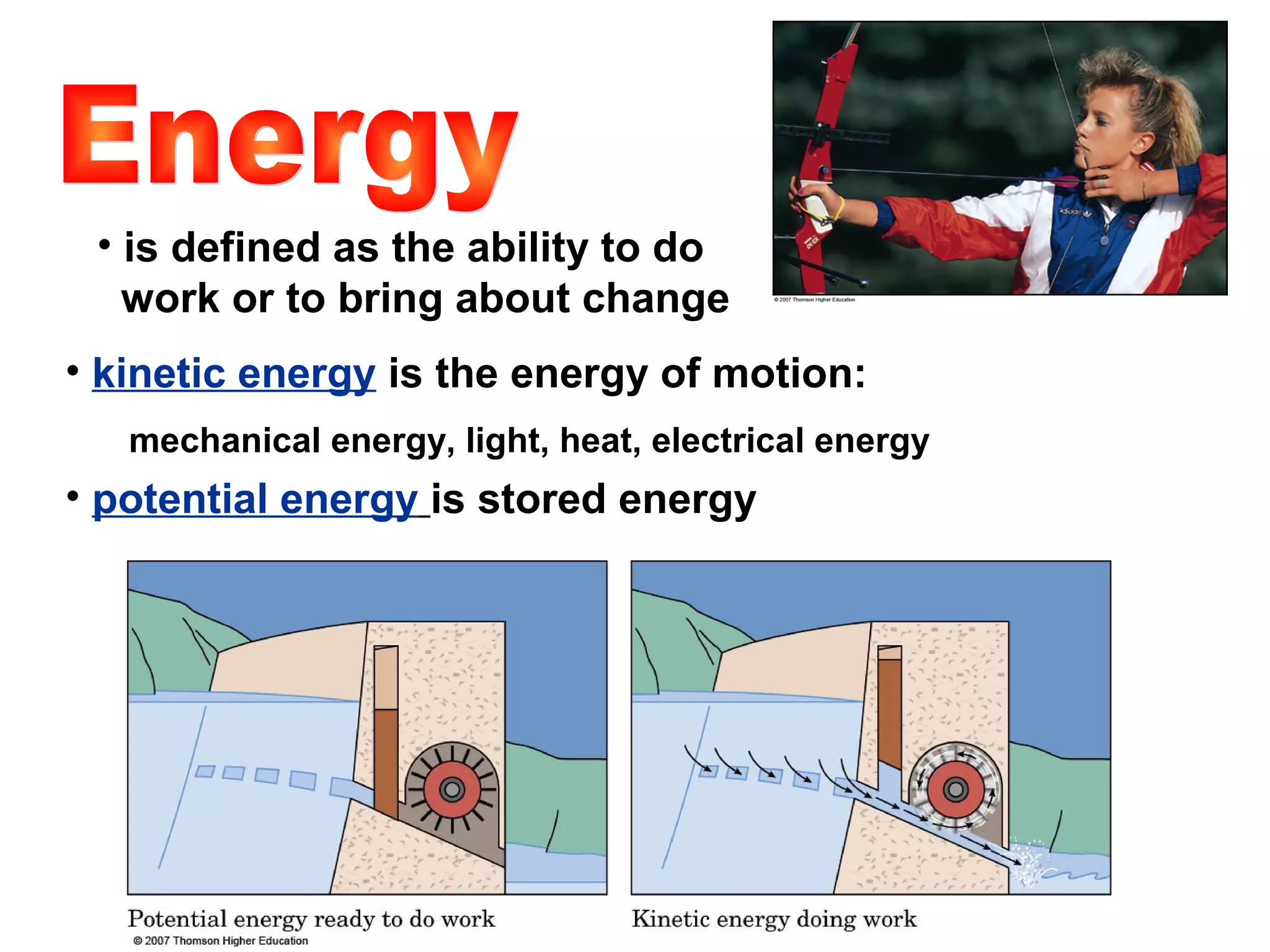
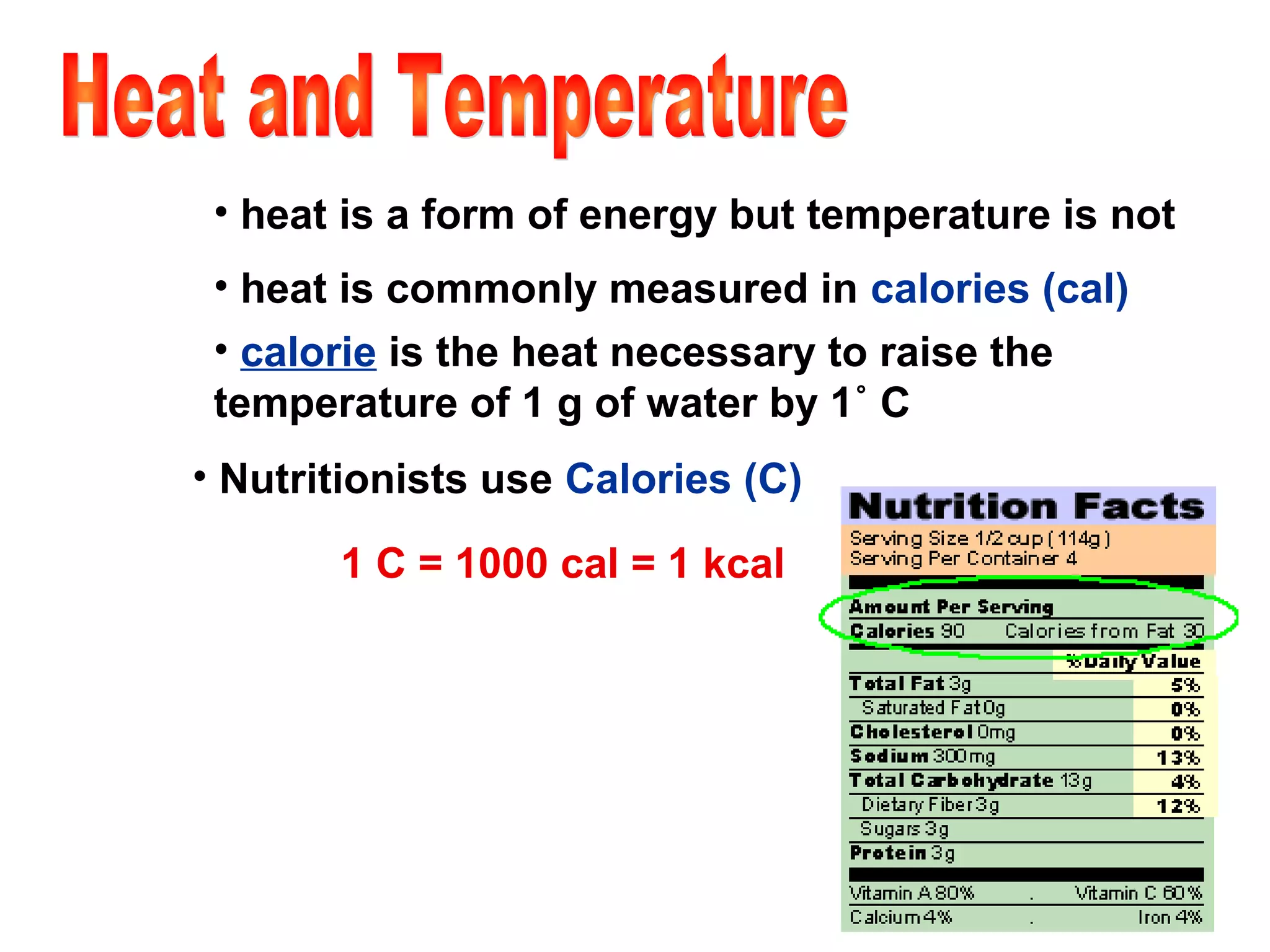
3) Energy exists in various forms and can be transformed from one to another but not created or destroyed. Chemical energy is stored in chemical bonds and released during chemical reactions.