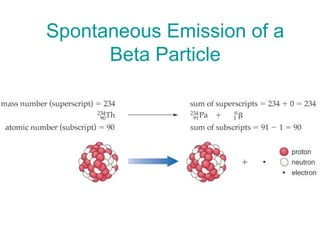
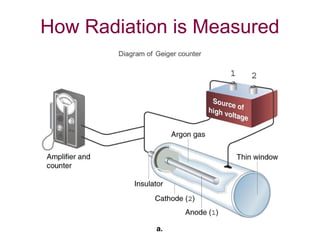
The document discusses the nature of radioactivity including the three main types of nuclear radiation (alpha, beta, gamma) and their properties. It describes different types of nuclear decay including alpha emission, beta emission, gamma emission, electron capture, and positron emission. Examples of radioactive isotopes used in dating and medicine are provided along with information on half-life, units of radiation measurement, and applications of radioisotopes. Review questions at the end assess understanding of nuclear equations, half-life calculations, and the inverse square law of radiation intensity.