This document summarizes key concepts about acids and bases from a chemistry textbook chapter:
1. It defines acids as substances that produce H+ ions in aqueous solution and bases as substances that produce OH- ions. Strong acids and bases fully dissociate while weak ones partially dissociate.
2. The pH scale is introduced and used to classify solutions as acidic, basic or neutral. Henderson-Hasselbalch equation relates pH, pKa, and concentrations of weak acids and their conjugate bases in buffer solutions.
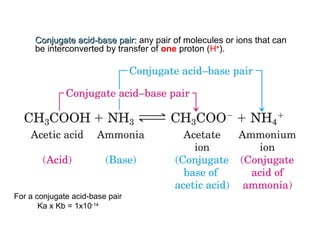
3. Key acid-base concepts covered include Brønsted-Lowry definitions, conjugate acid-base pairs, acid-base equilibria, self-ionization of water,










![Many bases like KOH, NaOH, Mg(OH)2, and Ca(OH)2] disassociate in
water producing OH-
ions (basic solution):
Other bases produce OH-
by reacting with water molecules, here shown
for ammonia:](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-12-320.jpg)






![Equilibrium/Ionization Constants
When a weak acid, HA, dissolves in water
the equilibrium constant expression, Keq, for this ionization is:
Water is the solvent and its concentration changes very little when we add HA,
we treat [H2O] as a constant equal to 1000 g/L or 55.5 mol/L. We combine the
two constants to give the acid ionization constant, Ka:](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-19-320.jpg)










![Self-Ionization of Water
14-
3
2
2
10x1.0
]][[][
=
== −+
w
w
K
OHOHOHKK
Compare to [H2O] = 55.5 mol/L
-log[1.0 x 10-7
] = 7= pH water](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-31-320.jpg)
![The product of [H3O+
] and [OH-
] in any aqueous solution is
equal to 1.0 x 10-14
.
If we add 0.010 mol of HCl to 1.00 liter of pure water, in
this solution [H3O+
] = 0.010 or 1.0 x 10-2
. This means that
the concentration of hydroxide ion is:
wKOHOH ==−+ -14
3 10x1.0]][[](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-32-320.jpg)
![pH and pOH
Because hydronium ion concentrations for most solutions
are numbers with negative exponents, we express these
concentrations as pH:
pH = -log [H3O+
]
Acidic solution:Acidic solution: pH < 7.0.
Basic solution:Basic solution: pH > 7.0.
Neutral solution:Neutral solution: pH = 7.0.](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-33-320.jpg)

![if we know the pH of an aqueous solution, we can easily
calculate its pOH:
pOH = -log[OH-
]
the ion product of water, Kw, is 1.0 x 10-14
taking the logarithm of this equation gives:
pH + pOH = 14](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-35-320.jpg)

![1. acidic.
2. basic.
3. neutral.
4. radioactive.
If a solution has a [OH-
] of 4.87 x 10-9
M, then the
solution is classified as:](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-37-320.jpg)





![pH = pKa
+ log
[CO3
2-
]
= - log (4.8e-11) + log
0.310
= 10.16
[HCO3
-
] 0.443
Substituting these values in the equation gives:
A buffer solution is 0.443 M in KHCO3 and 0.310 M in K2CO3.
If Ka for HCO3
-
is 4.8e-11
, what is the pH of this buffer
solution?](https://image.slidesharecdn.com/chapter8-181029212403/85/Chapter-8-43-320.jpg)