The document discusses different types of hydrocarbons:
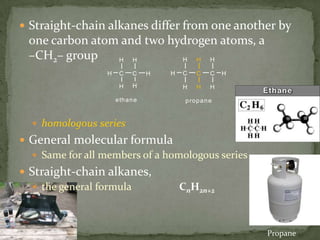
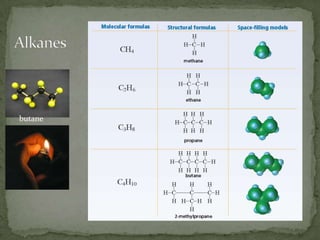
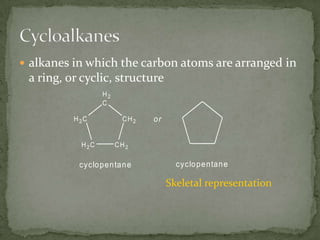
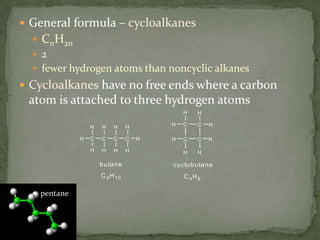
- Saturated hydrocarbons like alkanes have only single carbon-carbon bonds. Unsaturated hydrocarbons have double or triple bonds.
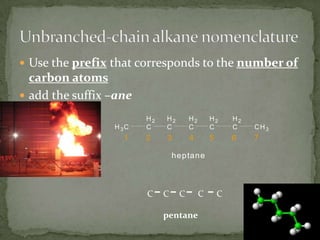
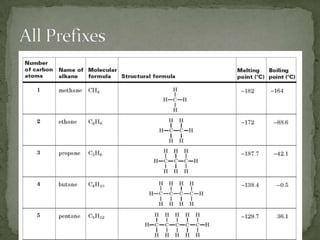
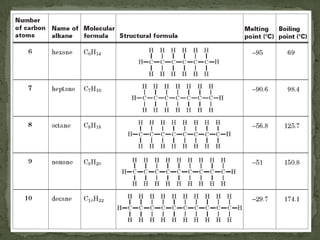
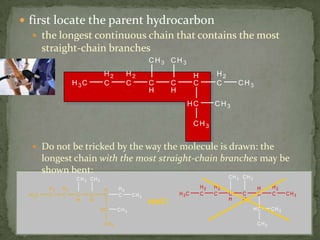
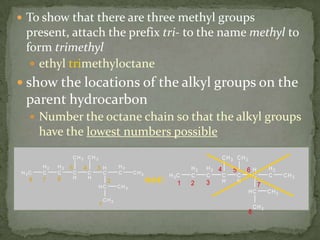
- Alkanes are named using Greek or Latin prefixes to indicate the number of carbon atoms and the suffix "-ane". Branched alkanes use alkyl groups.
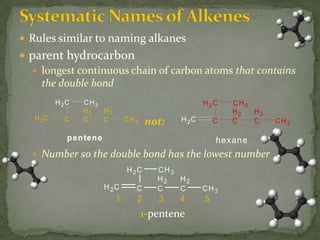
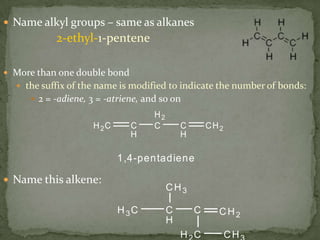
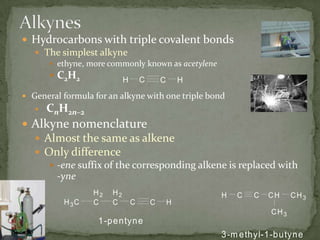
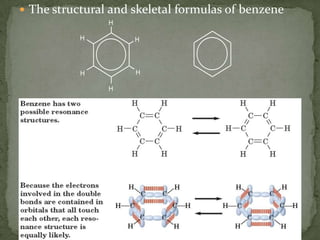
- Alkenes contain double bonds and are named similarly but use the suffix "-ene". Alkynes have triple bonds and use "-yne". Aromatic hydrocarbons have delocalized electrons in six-carbon rings like benzene.