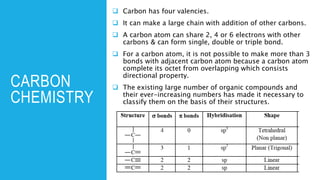
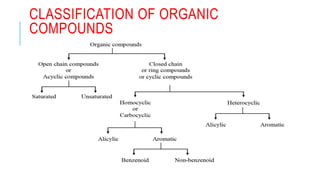
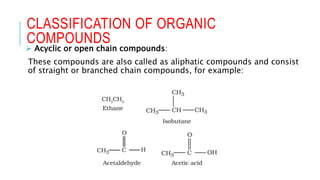
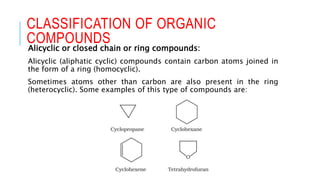
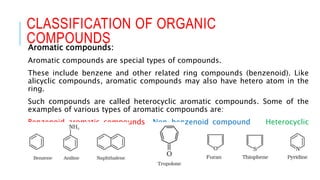
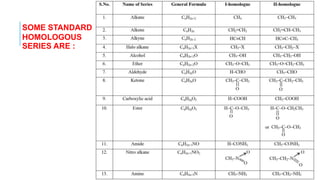
The document discusses the classification of organic compounds. Organic compounds are made up of carbon chains that can be open, closed in rings, or aromatic. Organic compounds can be classified based on their structure as acyclic/open chain, alicyclic/closed chain, or aromatic. They can also be classified based on functional groups into homologous series that have the same functional group but differ by CH2 units, such as alkanes, alkenes, alkynes, and others. The classification schemes are important for understanding the variety of organic compounds.