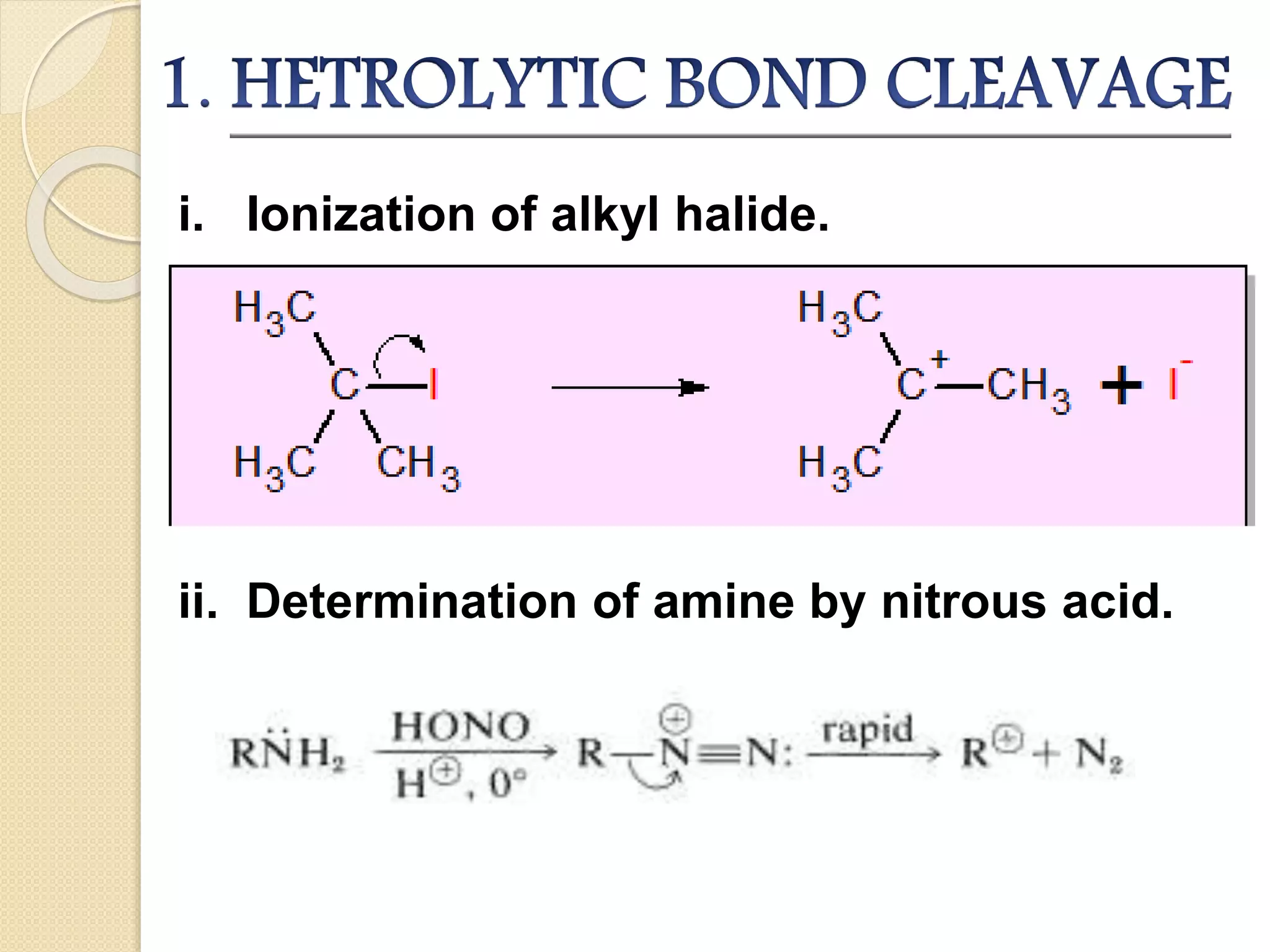
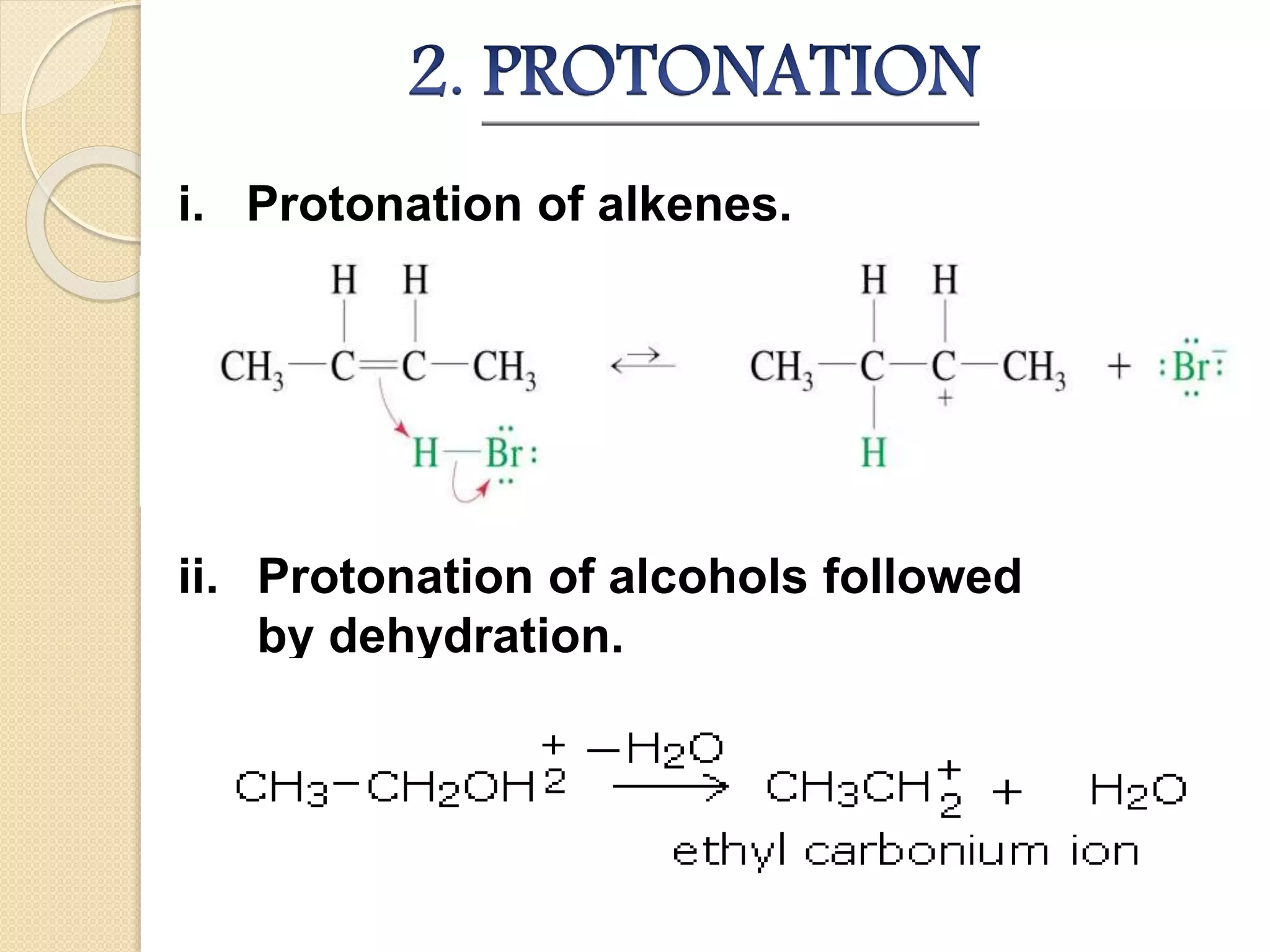
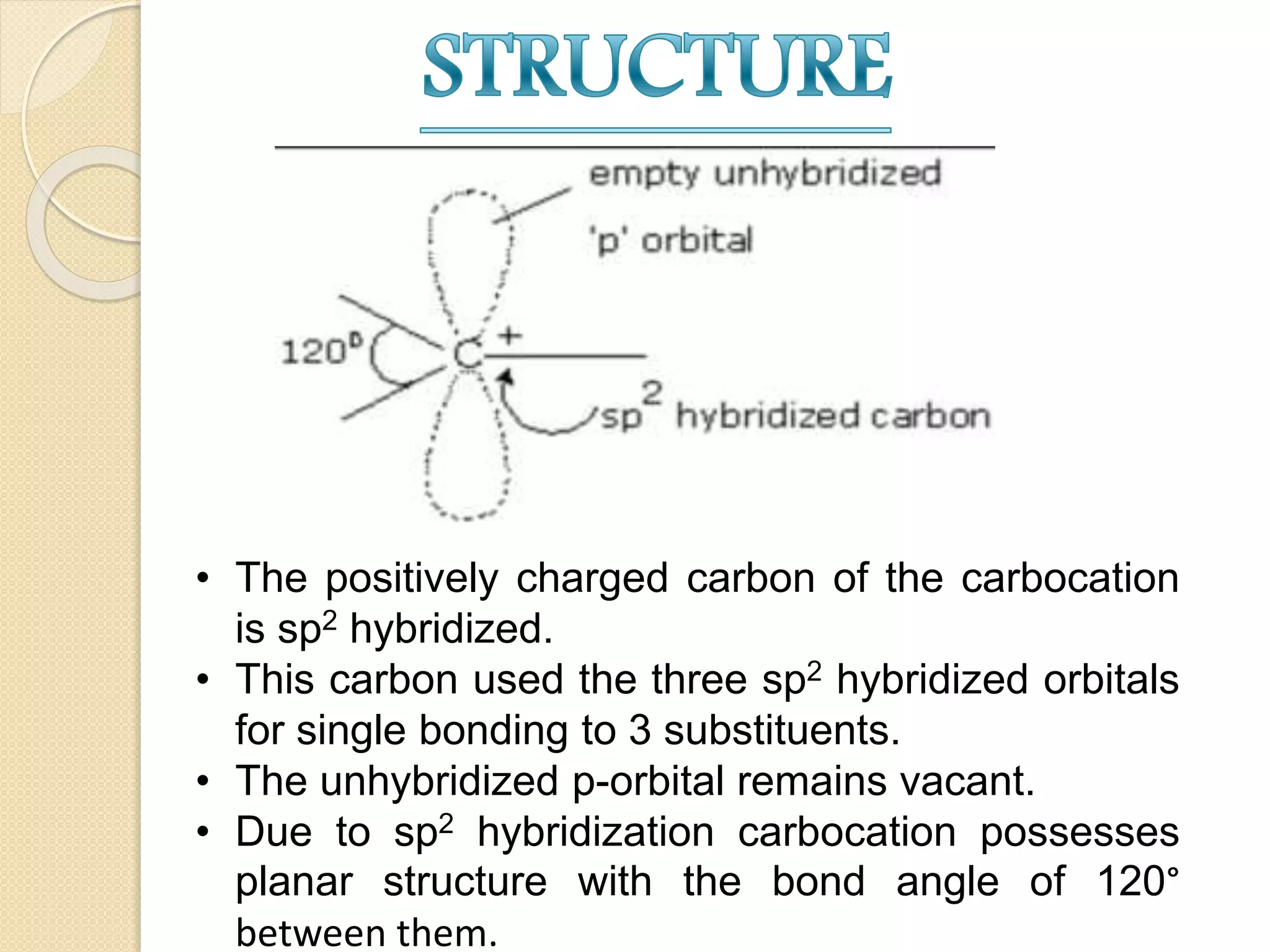
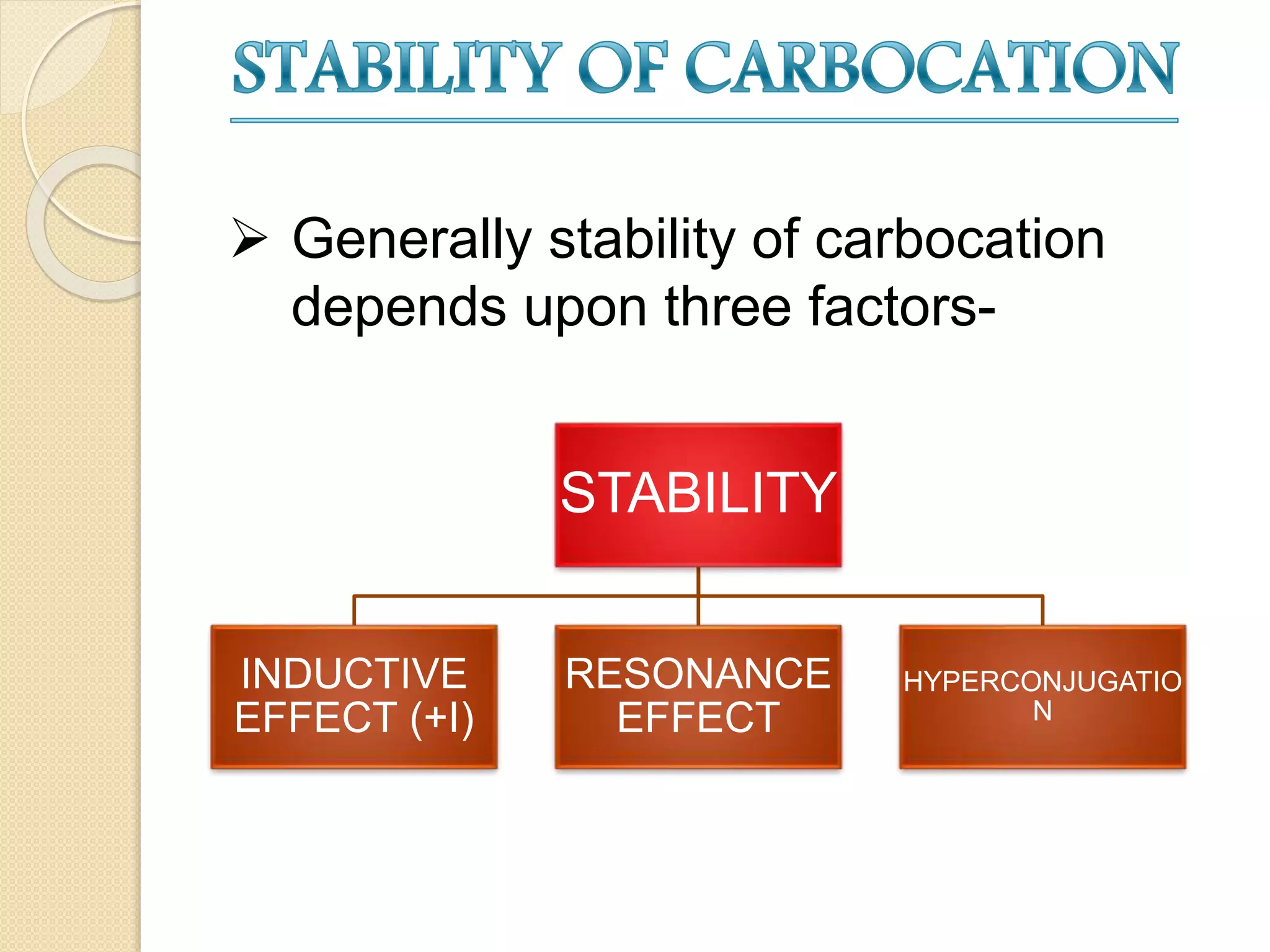
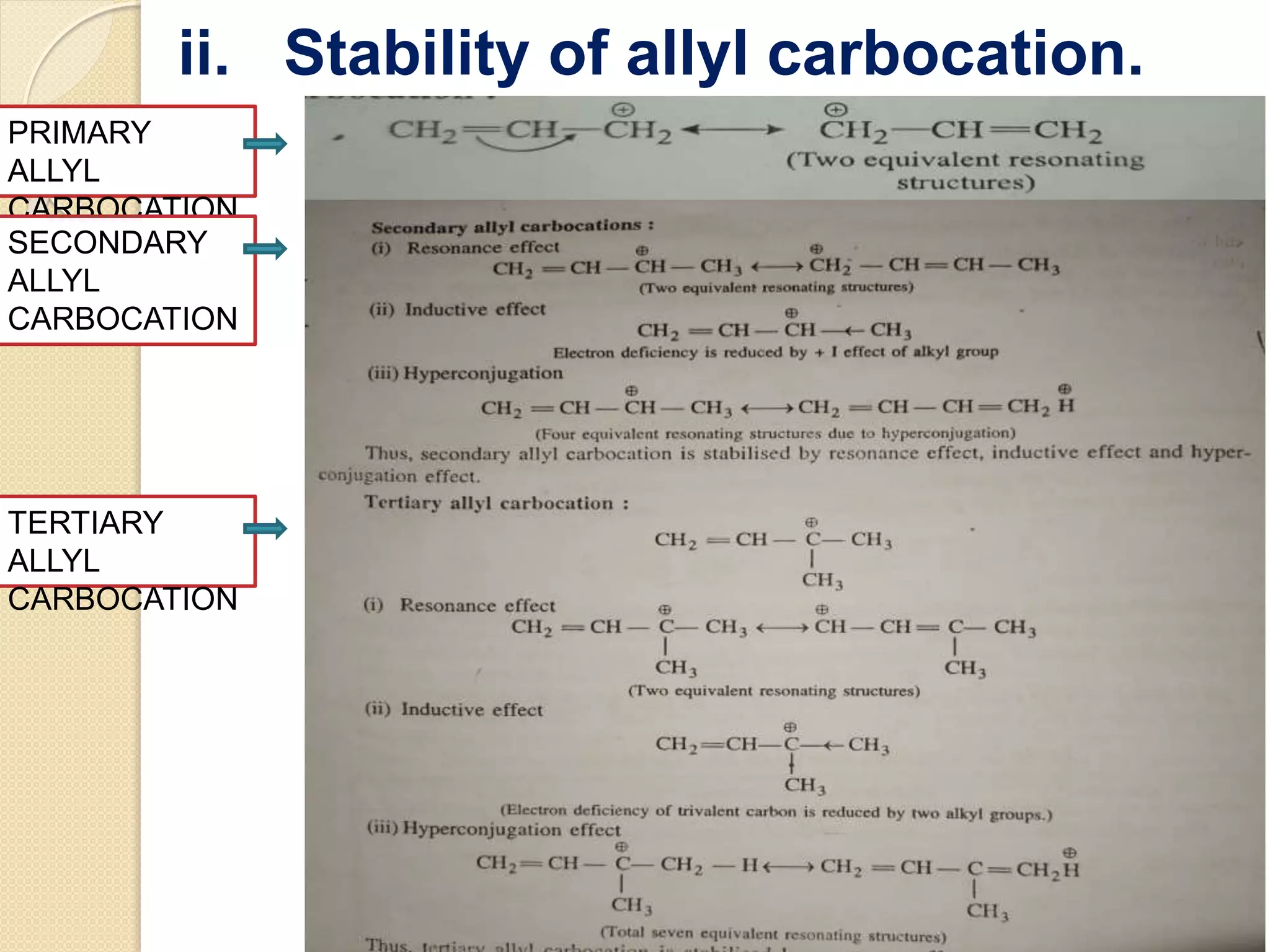
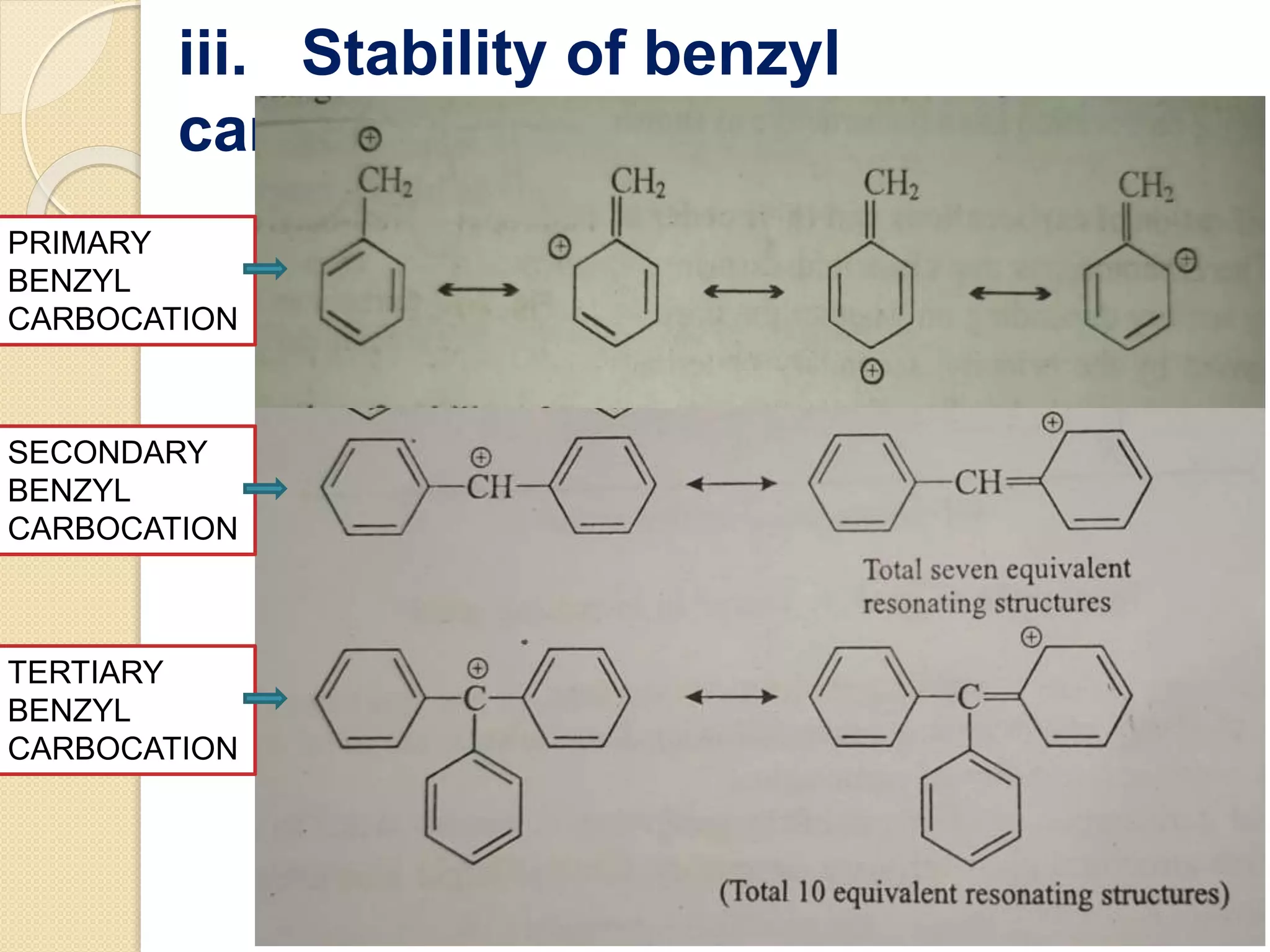
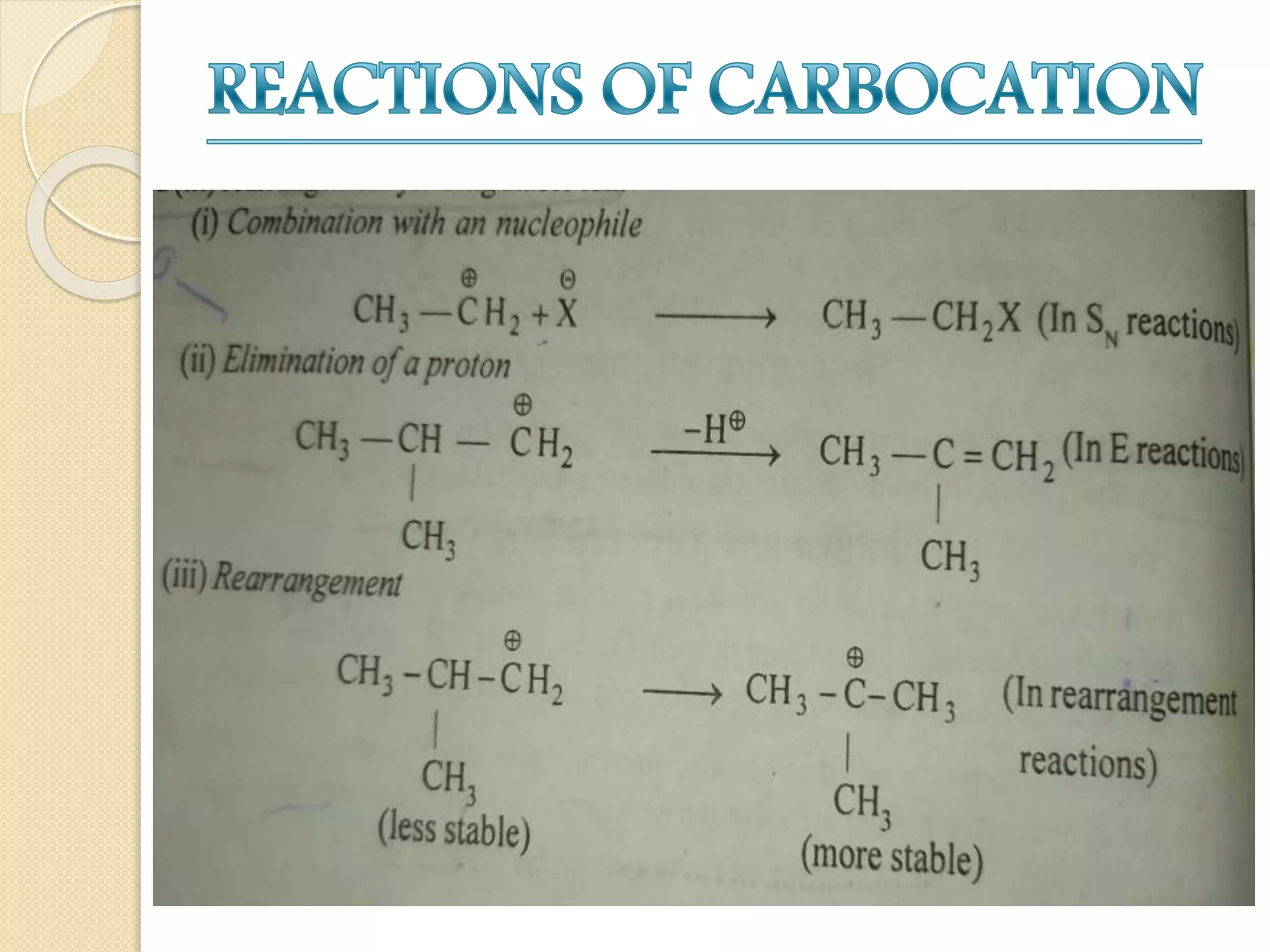
This document discusses carbocations, organic species with a positively charged carbon atom bearing only six electrons, which are sp2 hybridized and highly reactive intermediates. It covers their formation, structure, stability influenced by inductive effects, resonance, and hyperconjugation, as well as applications such as ionization of alkyl halides and protonation of alkenes. References for further reading are also provided, including academic books and online resources.