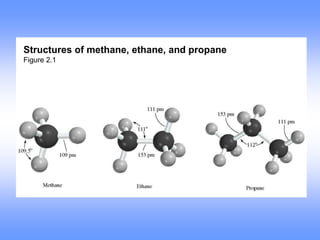
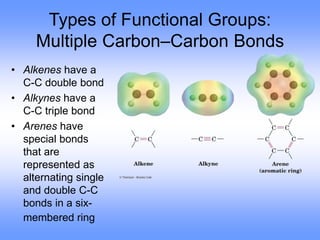
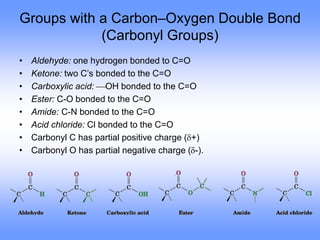
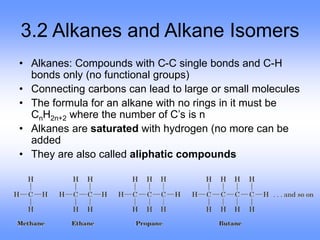
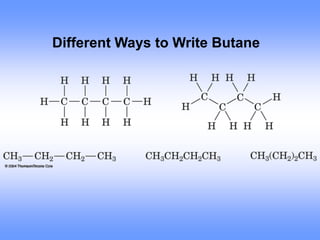
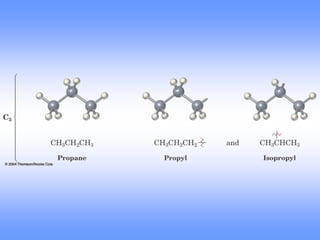
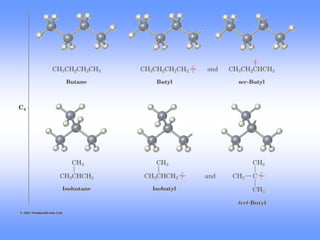
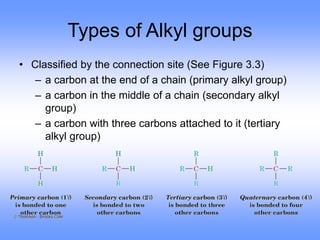
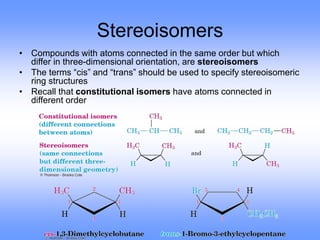
This document discusses organic compounds and their functional groups. It begins by introducing families of organic compounds and noting that they can be grouped by their common structural features. It then focuses on describing various functional groups, including their structures, properties, and common reactions. Specific functional groups discussed include alkanes, alkenes, alkynes, aromatics, alcohols, ethers, amines, thiols, aldehydes, ketones, carboxylic acids, and esters. The document also covers nomenclature rules for naming organic compounds.