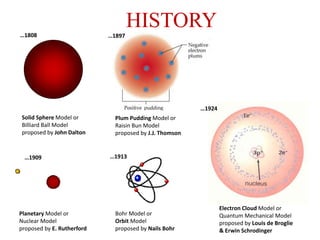
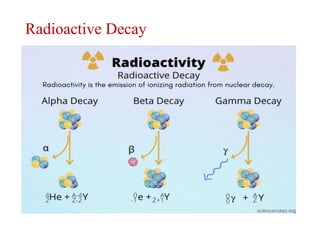
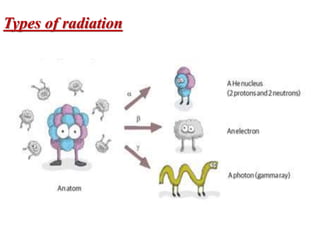
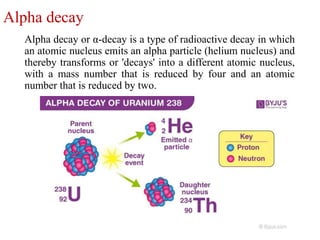
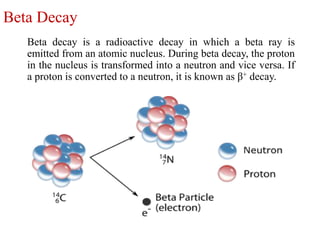
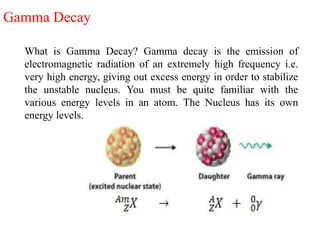
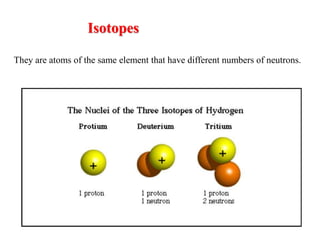
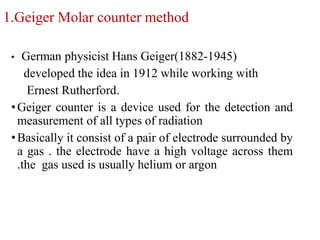
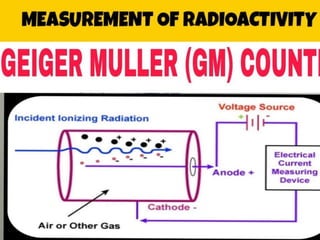
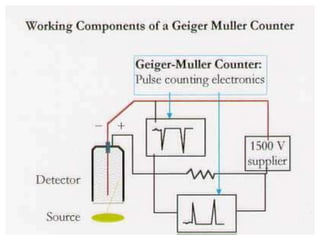
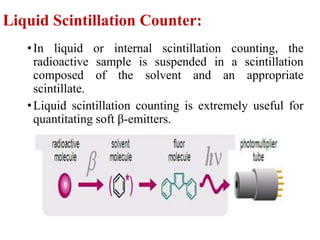
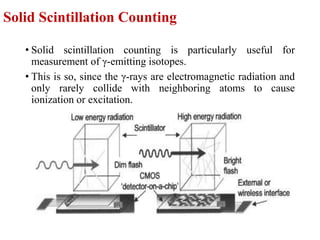
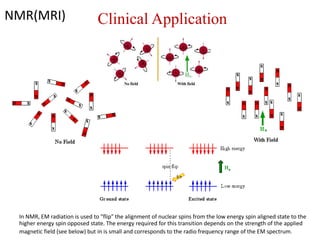
The document provides a comprehensive overview of radioactivity, covering its definition, types of radioactive decay (alpha, beta, gamma), and methods for measuring radioactivity, including Geiger counters and scintillation counters. It also discusses sources of radioactivity, clinical applications like NMR and CT imaging, and the advantages of utilizing radioactive materials. References to relevant literature and the historical development of atomic models are included.