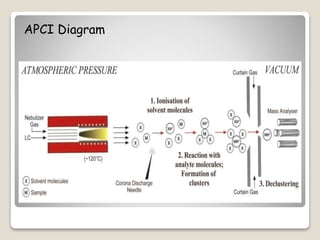
Atmospheric Pressure Chemical Ionization (APCI) is an ionization method that uses ion-molecule reactions to ionize samples, facilitated by a nebulization process and corona discharge. This method is beneficial for less-polar compounds and provides an excellent interface for liquid chromatography-mass spectrometry (LC/MS). However, APCI is sensitive to contaminants and has relatively low ion currents and complex hardware requirements.