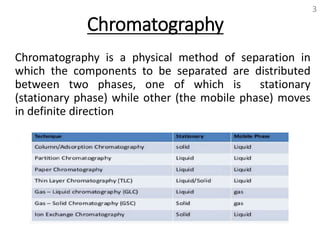
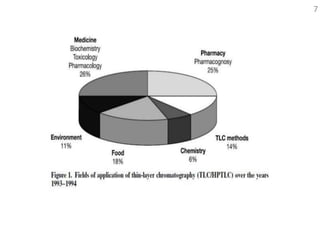
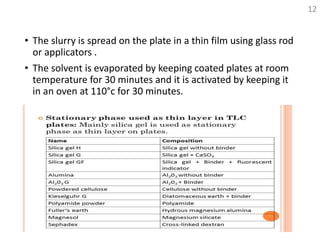
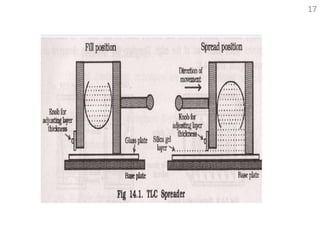
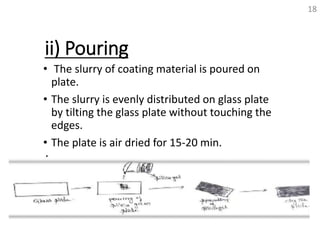
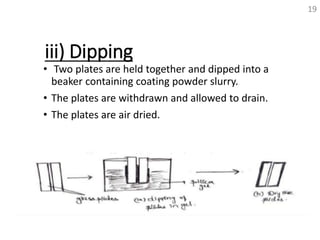
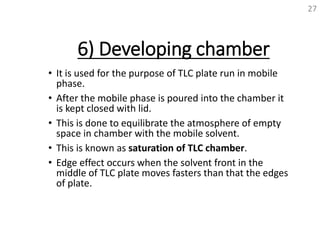
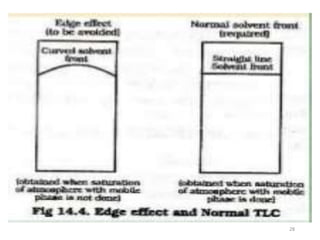
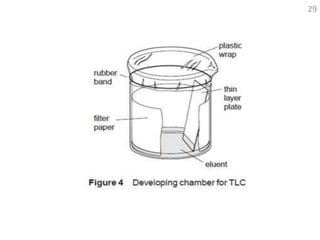
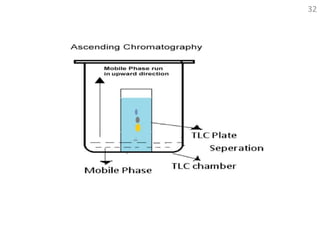
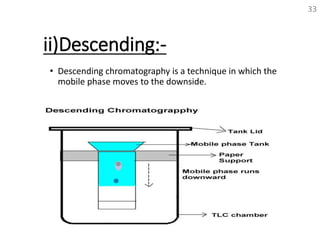
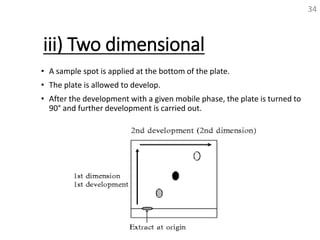
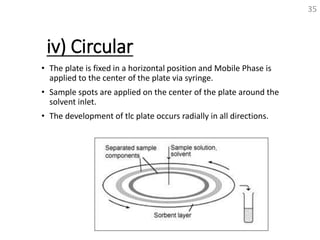
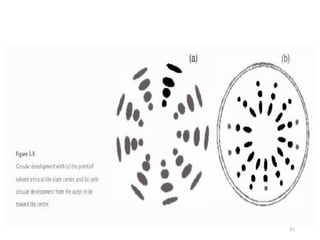
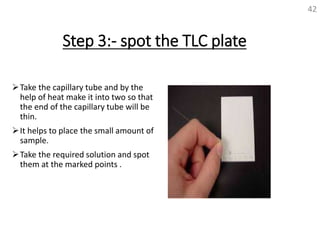
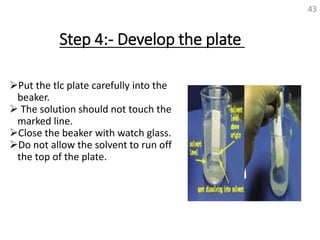
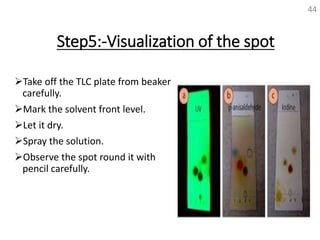
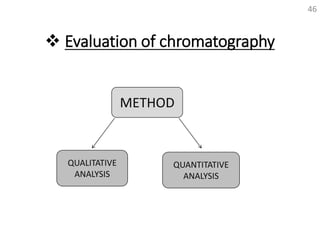
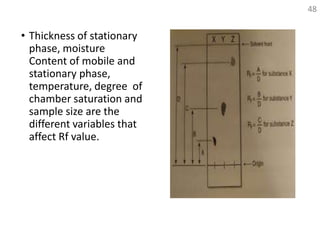
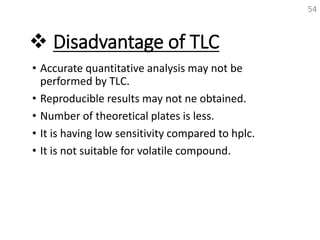
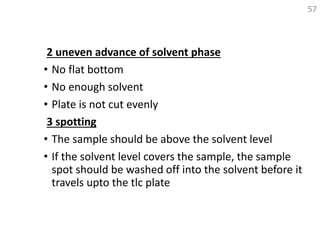
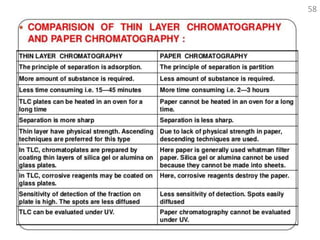
Thin layer chromatography (TLC) is a technique used to separate mixtures by distributing the components between a stationary phase and a mobile phase. The document discusses the principles, requirements, procedures, and applications of TLC. It explains that TLC involves applying a sample as a spot on a thin layer of adsorbent material like silica coated on a plate, then developing the plate in a mobile phase which separates the components by traveling up the plate at different rates based on their interactions with the phases. The document provides details on the materials, equipment, development techniques and evaluation of TLC.