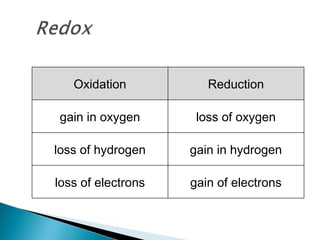
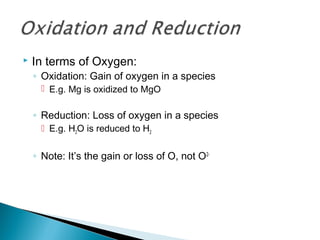
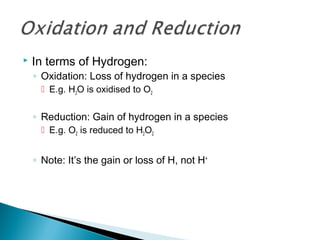
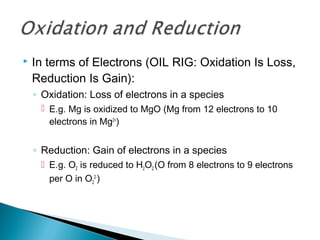
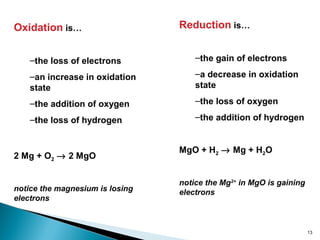
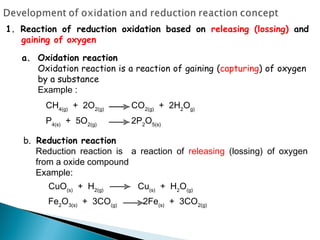
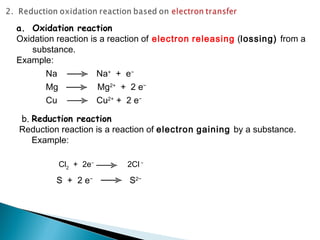
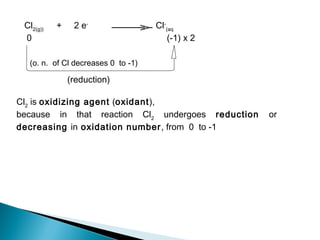
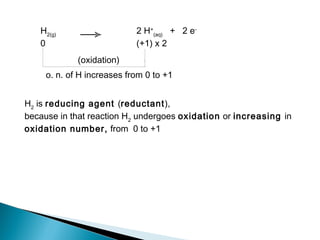
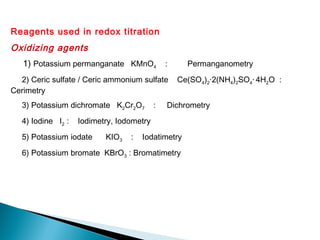
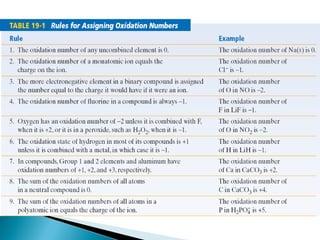
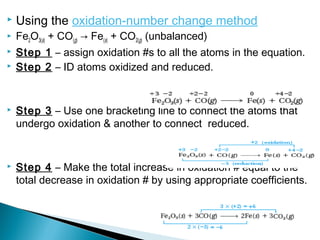
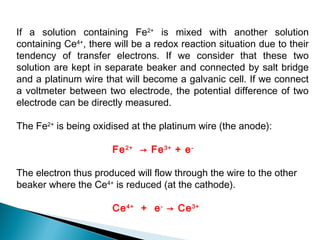
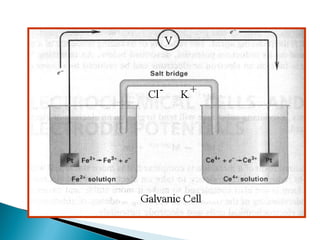
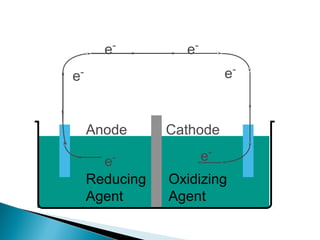
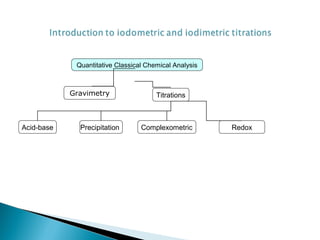
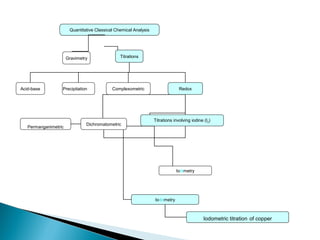
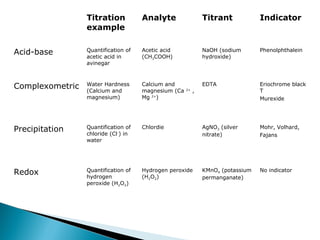
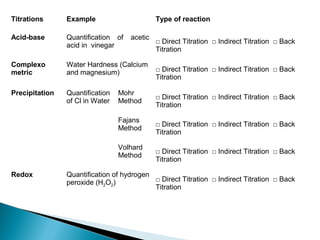
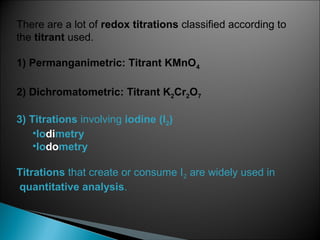
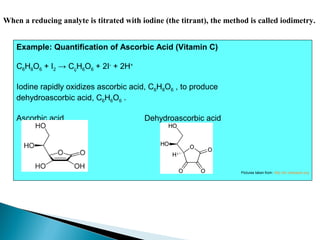
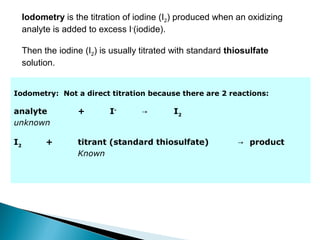
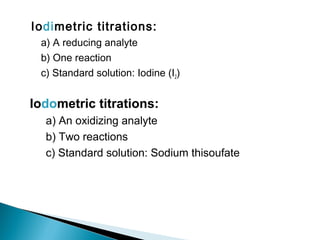
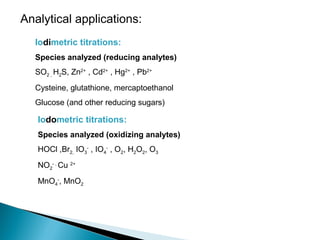
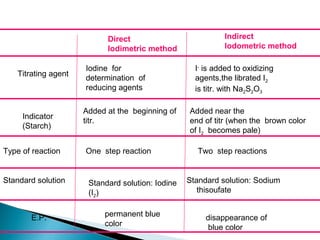
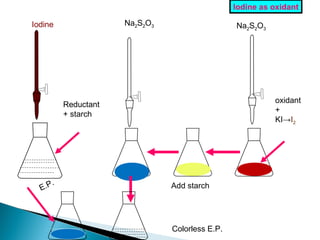
This document discusses oxidation-reduction (redox) reactions and concepts including definitions of oxidation and reduction in terms of gaining or losing electrons, oxygen, and hydrogen. It provides examples of redox reactions and identifies the oxidizing agent and reducing agent in reactions. It also discusses oxidation numbers and how to balance redox equations using the oxidation number change method. Finally, it discusses redox titrations and the specific methods of iodimetry and iodometry which involve the use of iodine as the titrant or analyte.