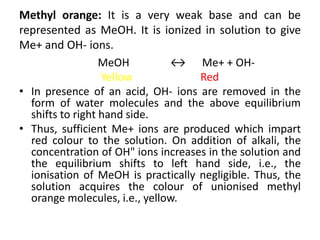
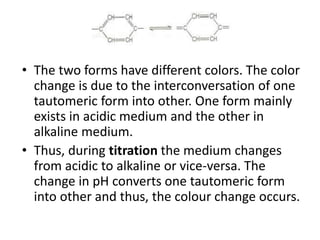
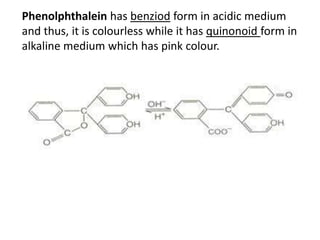
This document discusses the theory of acid-base indicators and their use in titrations. It explains that indicators change color within a certain pH range, with examples like methyl orange changing from pink to yellow between pH 3.2-4.5. Two theories are presented for how indicators work: Ostwald's theory, where color change is due to ionization, and the quinonoid theory, where indicators exist in different tautomeric forms with different colors. Phenolphthalein is used to illustrate Ostwald's theory, changing from colorless to pink as it ionizes more in alkaline conditions. Methyl orange is a weak base that changes from yellow to red depending on whether its ionized or unionized form
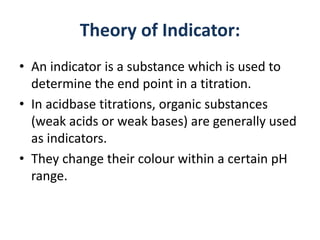


![Applying law of mass action
K = [H+][Ph- ]/[HpH]
• The undissociated molecules of phenolphthalein are
colourless while Ph- ions are pink in colour.
• In presence of an acid the ionisation of HPh is
practically negligible as the equilibrium shifts to left
hand side due to high concentration of H+ ions. Thus,
the solution would remain colourless.
• On addition of alkali, hydrogen ions are removed by
OH- ions in the form of water molecules and the
equilibrium shifts to right hand side.
• Thus, the concentration of Ph- ions increases in
solution and they impart pink colour to the solution.](https://image.slidesharecdn.com/theoryofindicator-190926073442/85/Theory-of-indicator-7-320.jpg)

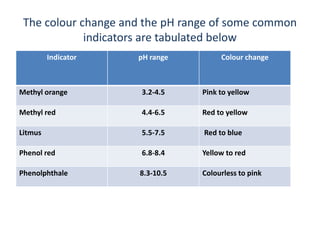
![Let us derive Handerson equation for an
indicator
HIn + H2O ↔ H+3O + In-
'Acid form' 'Base form‘
(Conjugate acid-base pair)
Kln = [ln][H+ 3O]/[HIn]
KIn = Ionization constant for indicator](https://image.slidesharecdn.com/theoryofindicator-190926073442/85/Theory-of-indicator-9-320.jpg)
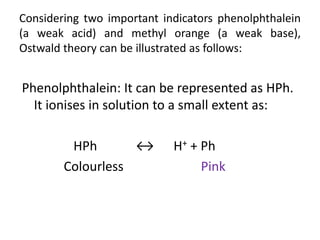
![[H+
3O] = KIn * [Hln]/ln-
pH = -log10 [H+
3O] = -log10[Kln] - log10[Hln]/[ln- ]
pH = pKIn + log10[ln- ]/[Hln]
(Handerson equation for indicator)
At equivalence point:
[In- ] = [HIn] and pH = pKIn](https://image.slidesharecdn.com/theoryofindicator-190926073442/85/Theory-of-indicator-10-320.jpg)