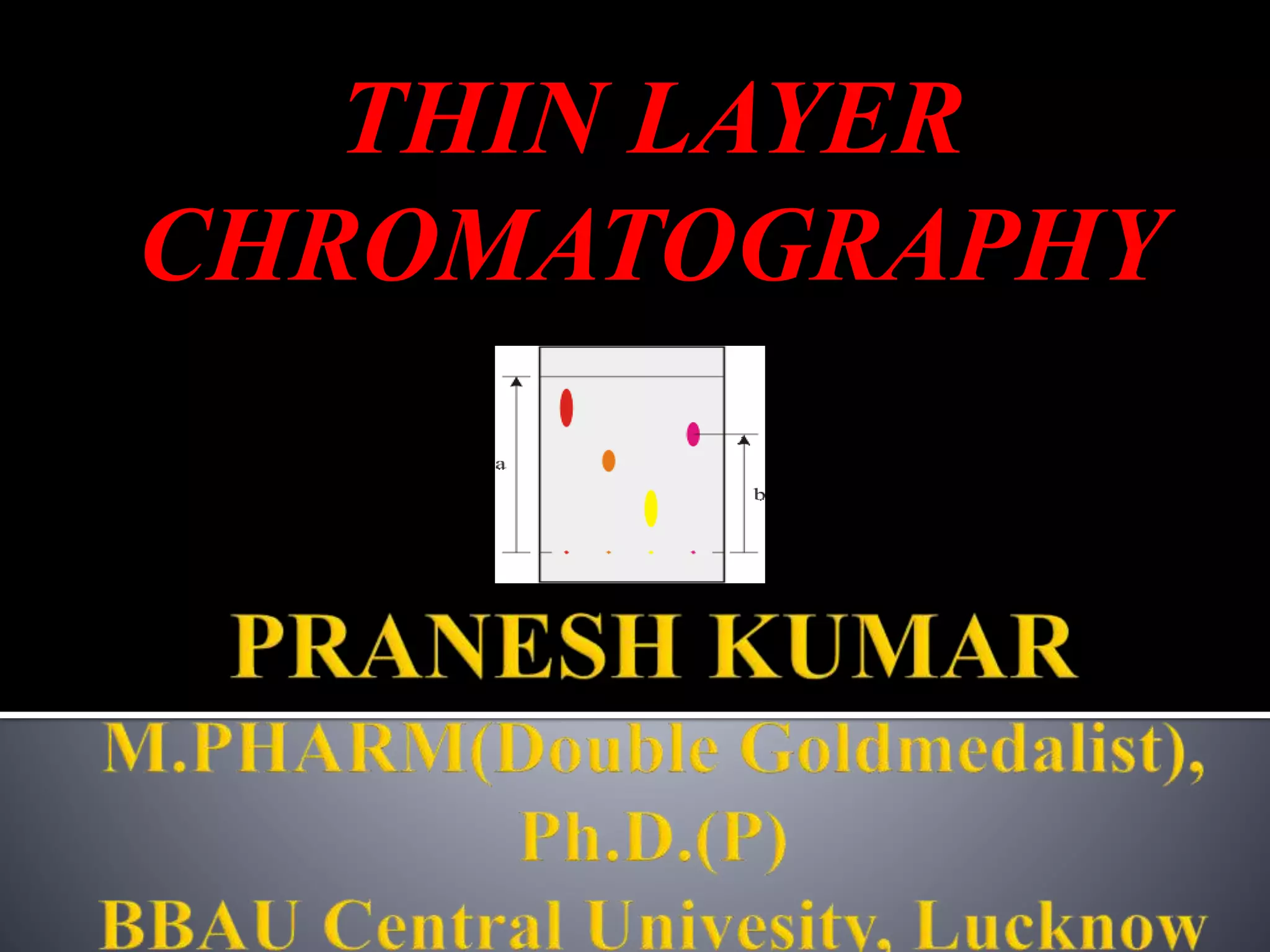
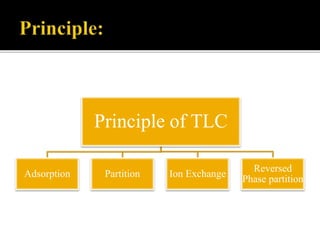
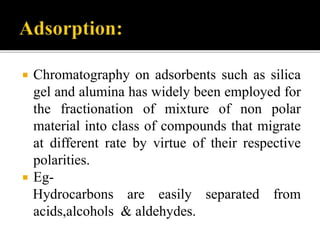
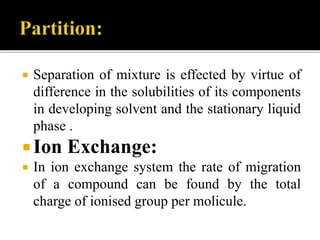
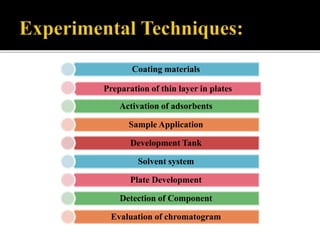
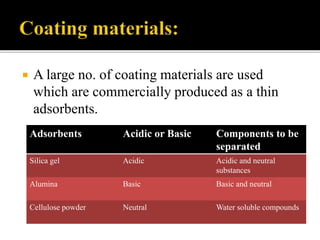
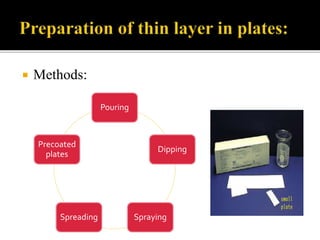
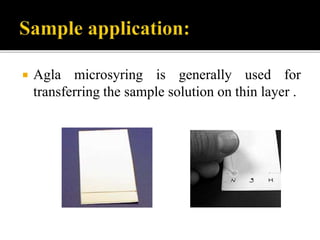
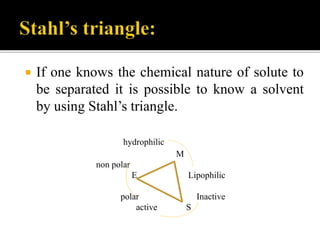
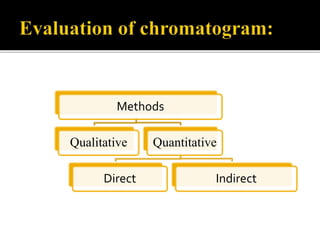
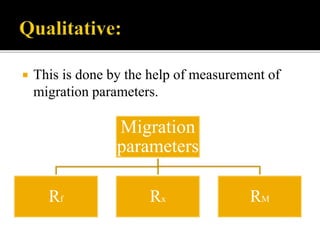
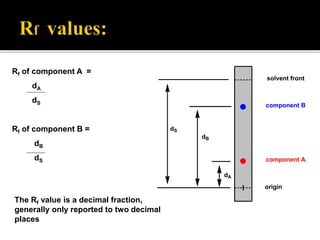
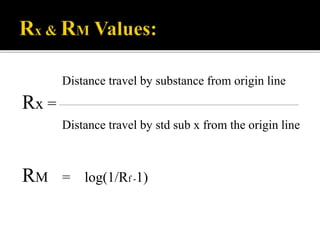
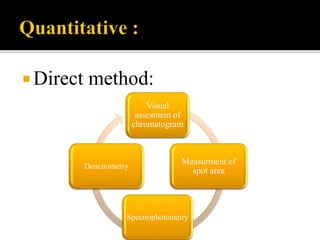
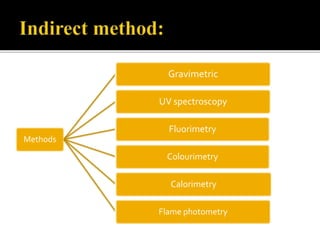
Thin layer chromatography (TLC) separates substances based on differential adsorption on inert solids using a mobile phase of solvent. The technique employs various adsorbents for fractionating mixtures, and different methods are used for applying samples and detecting components. TLC is utilized for purity checking, identifying compounds, and monitoring chemical reactions.