This document discusses various precipitation titration methods involving silver ions (Ag+). It describes three main methods:
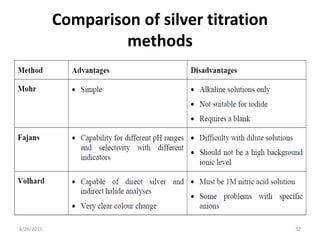
1) Mohr's method uses silver ions as the titrant and chromate ions as the indicator for titrating halide ions like chloride. Silver halide precipitates first, followed by silver chromate at the endpoint.
2) Volhard's method titrates silver ions with thiocyanate ions in acidic medium using ferric ions as the reddish-brown thiocyanate complex indicator.
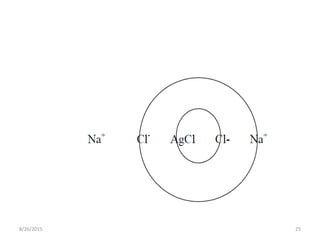
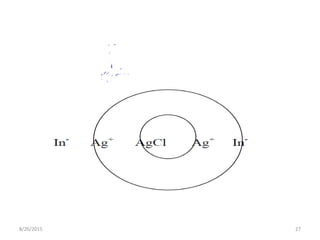
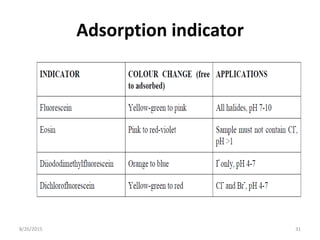
3) Fajan's method, or indicator adsorption method, involves adsorption of anionic dye indicators onto the precipitated silver halide particles. The intense color change at the












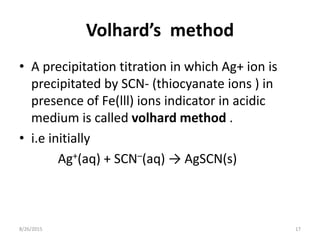
![• At end point
Fe3+(aq) + SCN–(aq) → [FeSCN]2+
reddish brown
• Here ,initially thiocyanate react with silver
ions and forms precipitate .at end point
,excess of thiocyanate (SCN-) react with Fe(lll)
and forms reddish brown complex which
indicate the end point of reaction .
8/26/2015 18](https://image.slidesharecdn.com/precipitationtitration-150826075122-lva1-app6891/85/Precipitation-titration-18-320.jpg)