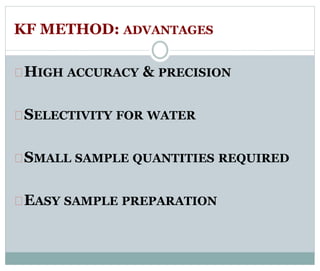
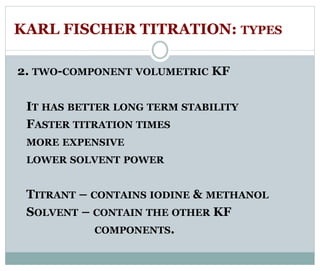
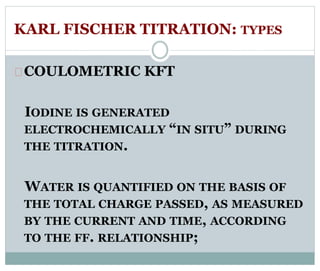
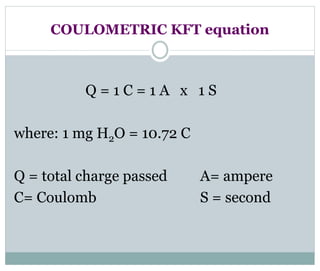
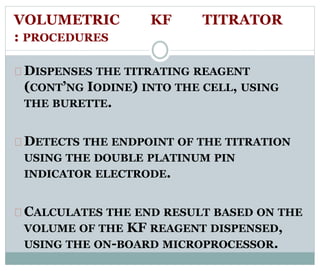
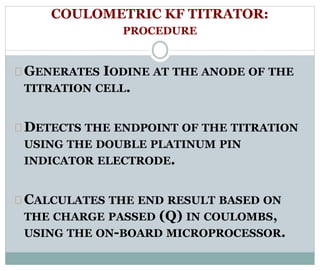
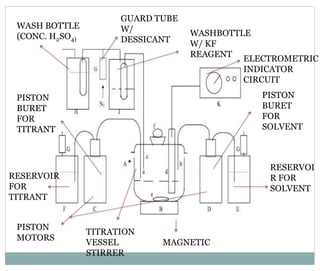
Chapter 6 discusses the Karl Fischer titration method for determining water content in various products, highlighting its principles, procedures, and advantages. The method involves a reaction between iodine and sulfur dioxide in an aqueous medium, allowing for precise water measurement with minimal sample size. It also contrasts Karl Fischer titration with the Loss on Drying method, emphasizing its specificity and accuracy in detecting water.





![KARL FISCHER REACTION
ROH + SO2 + R’N [R’NH]SO3R + H2O + I2 +
2R’N
ALCOHOL BASE ALKYLSULFITE SALT WATER IODINE
2[R’NH]I + R[N’H] SO4R
HYDROIODIC ACID SALT ALKYLSULFATE SALT](https://image.slidesharecdn.com/unit6-moistureanalysis-141006042945-conversion-gate02/85/Unit-6-Water-Content-Determination-and-Moisture-analysis-7-320.jpg)

![KARL FISCHER REACTION
THE REACTIVE ALCOHOL IS TYPICALLY;
1. METHANOL
2. 2[2-ETHOXYETHOXY] ETHANOL /
DIETHYELENE GLYCOL MONOETHYL
ETHER / DEGEE
3. OTHER SUITABLE ALCOHOL](https://image.slidesharecdn.com/unit6-moistureanalysis-141006042945-conversion-gate02/85/Unit-6-Water-Content-Determination-and-Moisture-analysis-10-320.jpg)