This document provides a summary of key concepts in oxidation-reduction (redox) reactions:
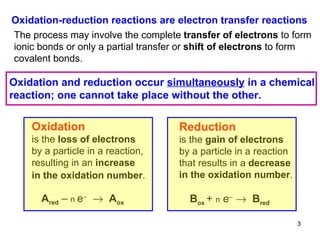
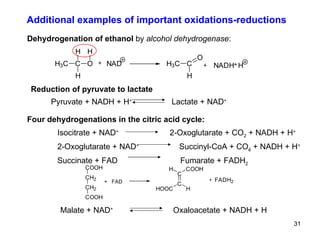
1) Redox reactions involve the transfer of electrons between chemical species, either through the complete transfer of electrons to form ionic bonds, or partial transfer to form covalent bonds. Oxidation is the loss of electrons and reduction is the gain of electrons.
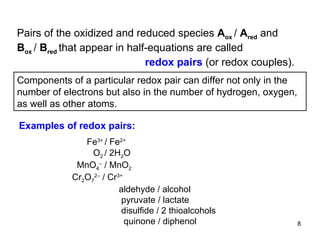
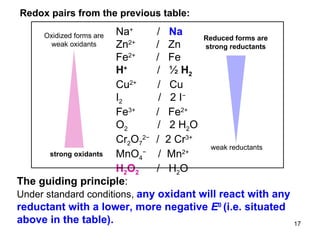
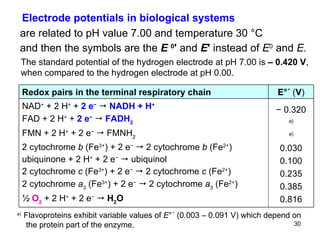
2) Redox pairs are couples of oxidized and reduced forms of elements that differ in their oxidation state. Common redox pairs include Fe3+/Fe2+, O2/H2O, and MnO4-/MnO2.
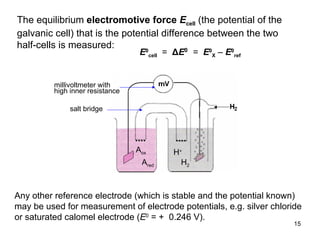
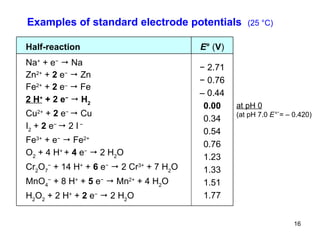
3) The standard electrode potential (E°) indicates the tendency of half-reactions to occur.



![Standard state of a half-cell: – both oxidized and reduced form of a redox pair at c = 1 mol/l – specified temperature , usually 25 °C – atmospheric pressure 101.3 kPa is important only when there is a gaseous component of the redox pair H 2 H + [ H + ] = 1 mol / l p H 2 = 101.3 kPa E 0 (H + /H) = 0.000 V (25 °C) Standard hydrogen electrode – reference electrode electrode - an inert metal A ox A red [ A ox ] = [ A red ] = 1 mol/l Half-cell to be measured in the standard state](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-14-320.jpg)
![If the difference Δ E 0 between both redox pairs is greater than 0.400 V, the reaction is irreversible (i.e. proceeds to completion) even under various non-standard concentrations of the reactants. If the difference between both E 0 is less than 0.400 V , then the reaction will reach equilibrium, the position of which depends on the initial concentrations of components of both redox pairs; the direction of such a reaction may be reversed . Electrode potentials E under non-standard conditions for a redox pair a A ox + n e – b A red Nernst equation RT n F ln E = E 0 + [ A ox ] a [ A red ] b [ A ox ] and [ A red ] relevant concentrations of reactants R = 8.314 kPa K –1 mol –1 F = 96 500 C mol –1 n = number of moles of electrons transferred E , E 0 el. potentials in volts](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-18-320.jpg)
![After expressing R , T (298 K), and F in numbers and transposing natural logarithm into decadic (ln x = 2.3 log x), the equation will take the form (in volts; t = 25 °C) The electrode poten t ial of half-cells at various concentrations of redox pair components can be calculated. On the contrary, the ratio of both redox pair components can be estimated from the measured values of electrode potentials. Galvanic cells (electrochemical cells) are two half-cells connected by an external conductor . This arrangement allows discharging of the cell , the spontaneous cell reaction (the sum of the chemical half-reactions). The electrons lost flow in the external circuit from the substance that is being oxidized in one of the half-cells to the substance that is being reduced in another half-cell till the cell reaches an equilibrium . The free energy resulting from the spontaneous reaction is released as electrical energy and/or heat. 0.059 n log E = E 0 + [ A ox ] a [ A red ] b](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-19-320.jpg)
![Before a conductive connection of the half-cells, the electromotive force of the galvanic cell E cell equals E cell = Δ E = E A – E B . The expected cell reaction is According to Nernst equation, The cell potential Δ E is sometimes described as the "driving force" of the cell reaction. It is related to the amount of electrical work that a cell can perform. After the external circuit is closed, the cell reaction is started. It goes on till the Δ E equals zero (the equilibrium state is reached) . A red + B ox A ox + B red the external circuit E cell = Δ E = Δ E 0 + RT n F ln [ A ox ] i [ B red ] i [ A red ] i [ B ox ] i (in volts, 25 °C)](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-20-320.jpg)
![A general rule suggests that the oxidized form of a redox pair with the more positive E is able to oxidize the reduced form of the redox pair with the less positive E. that iodine will Example: Redox reaction: Half-reactions: The oxidation of iodide to elemental iodine will not be complete , the difference Δ E 0 is less than 0.40 V. Under which condition is iodine able to oxidize Fe 2+ ? It follows from oxidize Fe 2+ to Fe 3+ only if there is much higher initial concentration of I 2 and Fe 2+ than of I – and Fe 3+ (the ratio of the products [I] i [Fe 2+ ] i and [I – ] i [Fe 3+ ] i must be greater than approx. 2500). Even so, the equilibrium will be reached after oxidation of a very small amount of Fe 2+ . Fe 3+ + e – Fe 2+ E 2 0 = 0.75 V I 2 + 2 e – 2 I – E 1 0 = 0.55 V Conventionally, the more positive E in the cell is described as E 2 . Fe 2+ + I 2 Fe 3+ + 2 I – The preferred reaction is then Fe 2+ + I 2 Fe 3+ + 2 I – ln [ Fe 3+ ] i [ I – ] i [ Fe 2+ ] i [ ½ I 2 ] i Δ E = Δ E 0 + RT n F](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-21-320.jpg)
![Relationship between the free energy change Δ G of redox reactions and the cell potential Δ E The cell potential Δ E is a measure of whether a redox reaction is spontaneous. F or the reaction A red + B ox -> A ox + B red it equals The Gibbs free energy change Δ G is the quite general measure of reaction spontaneity and equals the free energy to do useful work. For the reaction A + B -> C + D, The electrical work available from a redox reaction – Δ G is equal to electrochemical potential Δ E times the electrical charge q (equal to n F ) transferred in a redox reaction. If a redox reaction starts at the standard state, – G 0 = n F E 0 . Δ G = Δ G 0 + RT ln [A] ï [B] i [C] i [D] i ln Δ E = Δ E 0 + RT n F [A red ] ï [B ox ] i [A ox ] i [B red ] i – G = n F E ( J mol –1 )](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-22-320.jpg)
![Relationship beween Δ E 0 and the equilibrium constant K ln Δ E = 0 = Δ E 0 – RT n F [A red ] a eq [B ox ] c eq [A ox ] b eq [B red ] d eq The equilibrium constant K eq of a redox reaction a A red + c B ox b A ox + d B red is K eq = [ A red ] a eq [ B ox ] c eq [ A ox ] b eq [ B red ] d eq After the galvanic cell reaction reaches its equilibrium (the cell is discharged), Δ E equals zero ( E 1 = E 2 ). Δ E 0 = ln K and ln K = Δ E 0 After expressing R , T (298 K), and F in numbers and transposing natural logarithm into decadic (ln x = 2.3 log x), the equation will take the form log K = Δ E 0 (25 °C) RT n F n 0.059 RT n F](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-23-320.jpg)


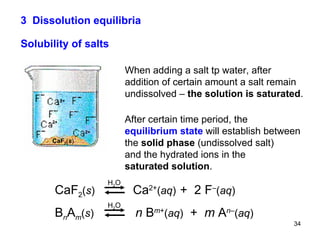
![is described by the equilibrium constant Because the activity (then also " concentration ") of any solid is taken as equal to unit ones and the amount of undissolved solid salt does not influence the ion concentration in a saturated solution, the simplified constant is called the solubility product K S : e.g., B n A m ( s ) n B m + ( aq ) + m A n – ( aq ) H 2 O The equilibrium state of a saturated solution CaF 2 ( s ) Ca 2+ ( aq ) + 2 F – ( aq ) H 2 O or [ B m + ] [ A n – ] K S (B n A m ) = [ Ca 2+ ] [ F – ] 2 K S (CaF 2 ) = K = [ B m + ] n [ A n – ] m [ B n A m ] [ Ca 2+ ] [ F – ] 2 [ CaF 2 ] K = or](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-35-320.jpg)
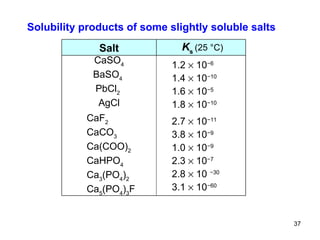
![The common ion effect An example: To a saturated AgCl solution ( K S = 1.7 10 –10 ) solution of hydrochloric acid is added until the chloride concentration [Cl – ] is 0.1 mol/l. The concentration of silver ion [Ag + ] in saturated solution equals 1.3 10 –5 . K S = [Ag + ] [Cl – ] = 1.7 10 –10 is the constant and remains the same no matter how the concentration [Cl – ] is changed. Then K S = 1.7 10 –10 = 0.1 x and x = [Ag + ] = 1.7 10 –9 after Cl – addition. In this case, a 10 000-fold reduction of Ag + ions in solution is shown. The extinct free Ag + ions were precipitated as AgCl. The common ion effect is the process, in which increasing the concentration of one of the ions in an equilibrium results in a decrease of the concentration of the other ions.](https://image.slidesharecdn.com/04redoxreactionsdissoln-precip-110914014811-phpapp01/85/04-redox-reactions__dissoln-__precip-41-320.jpg)