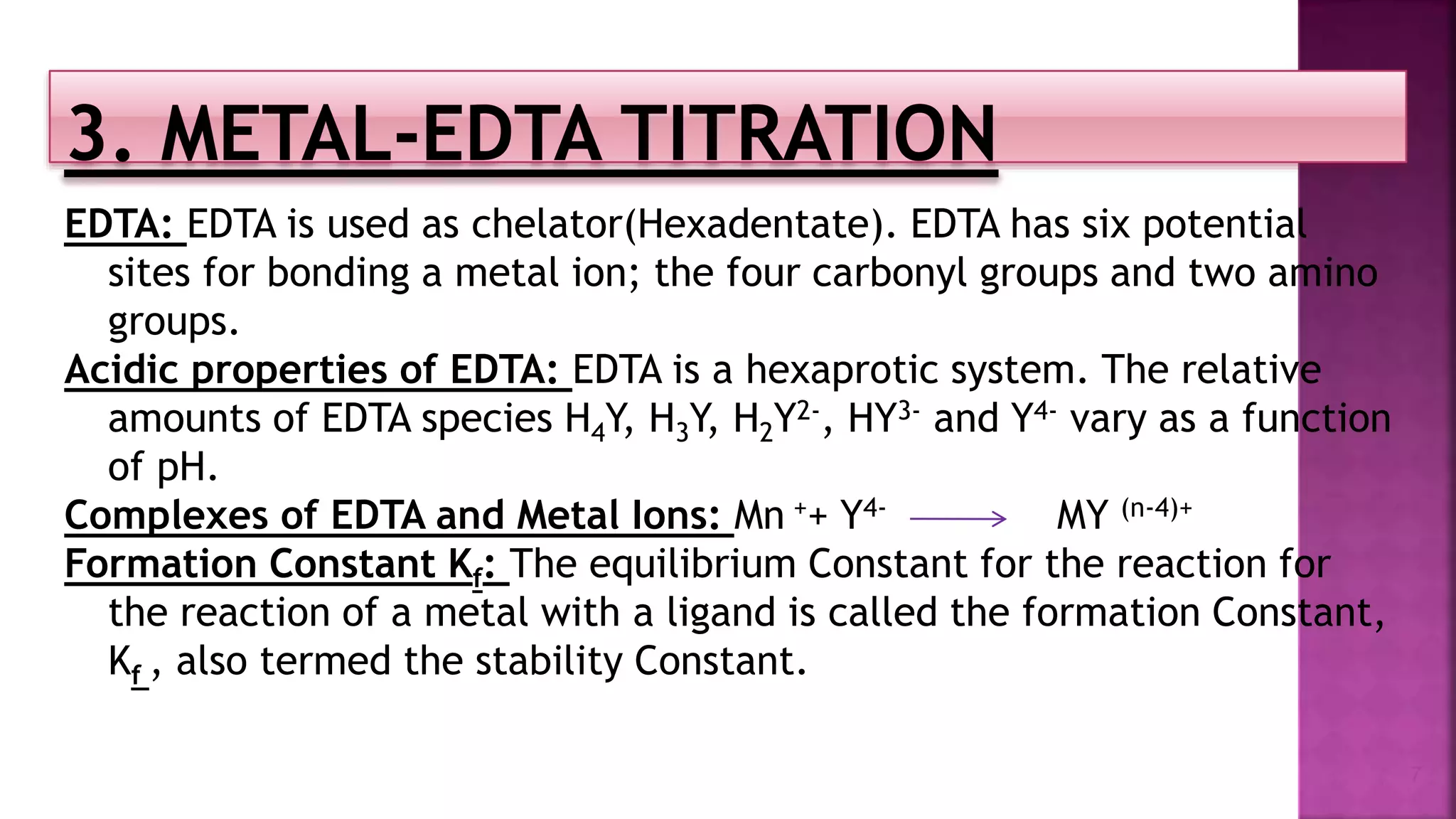
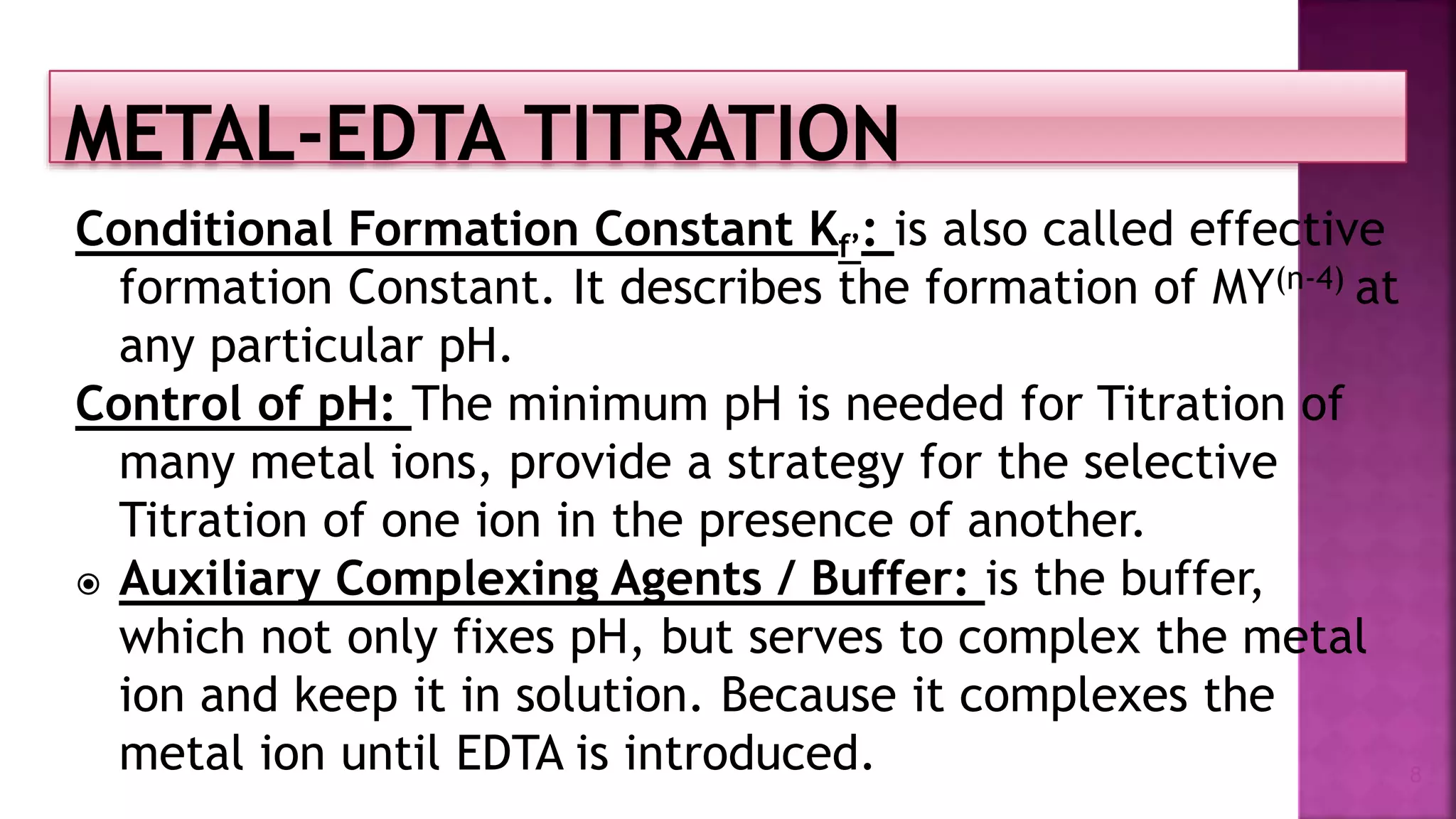
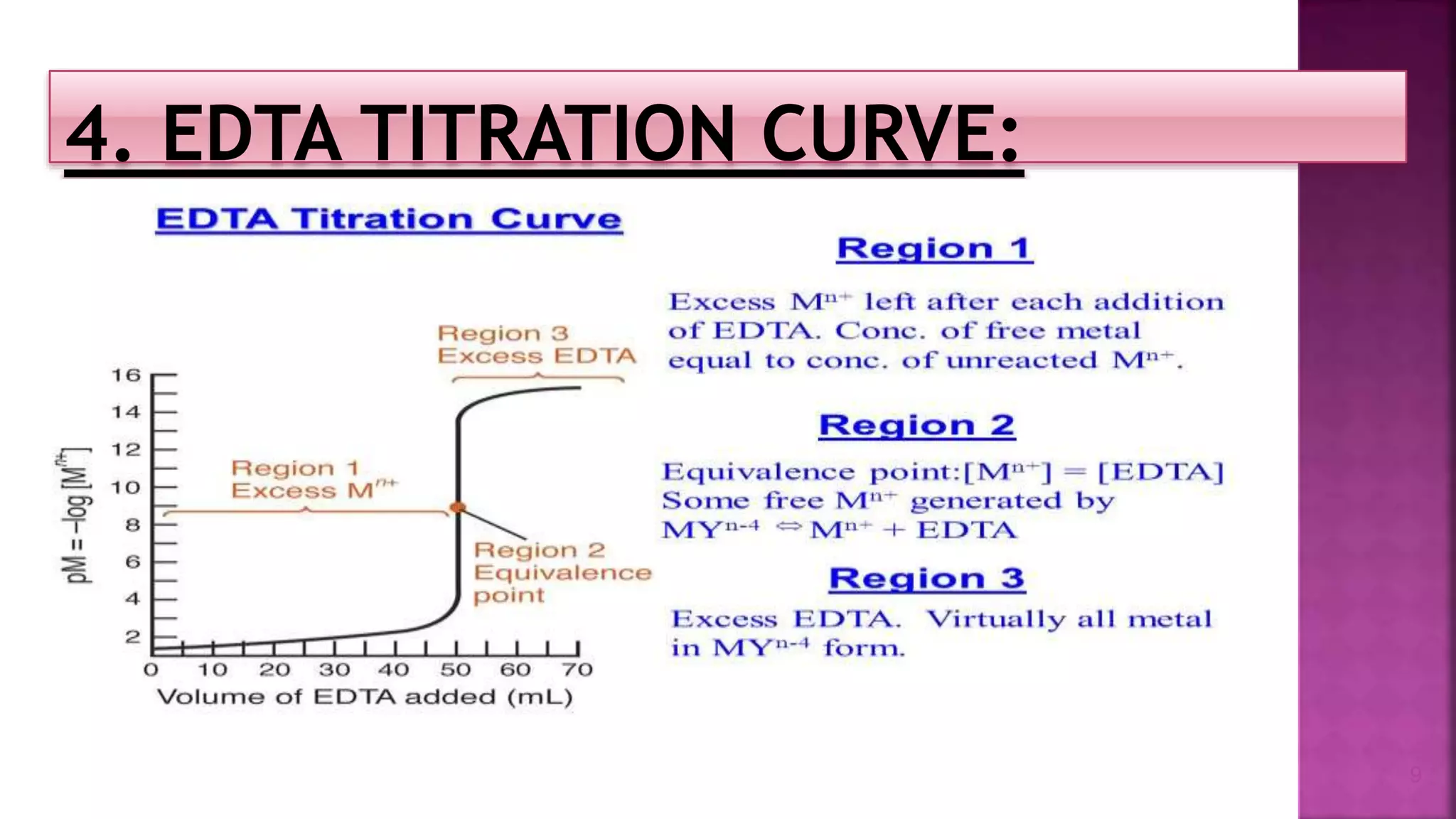
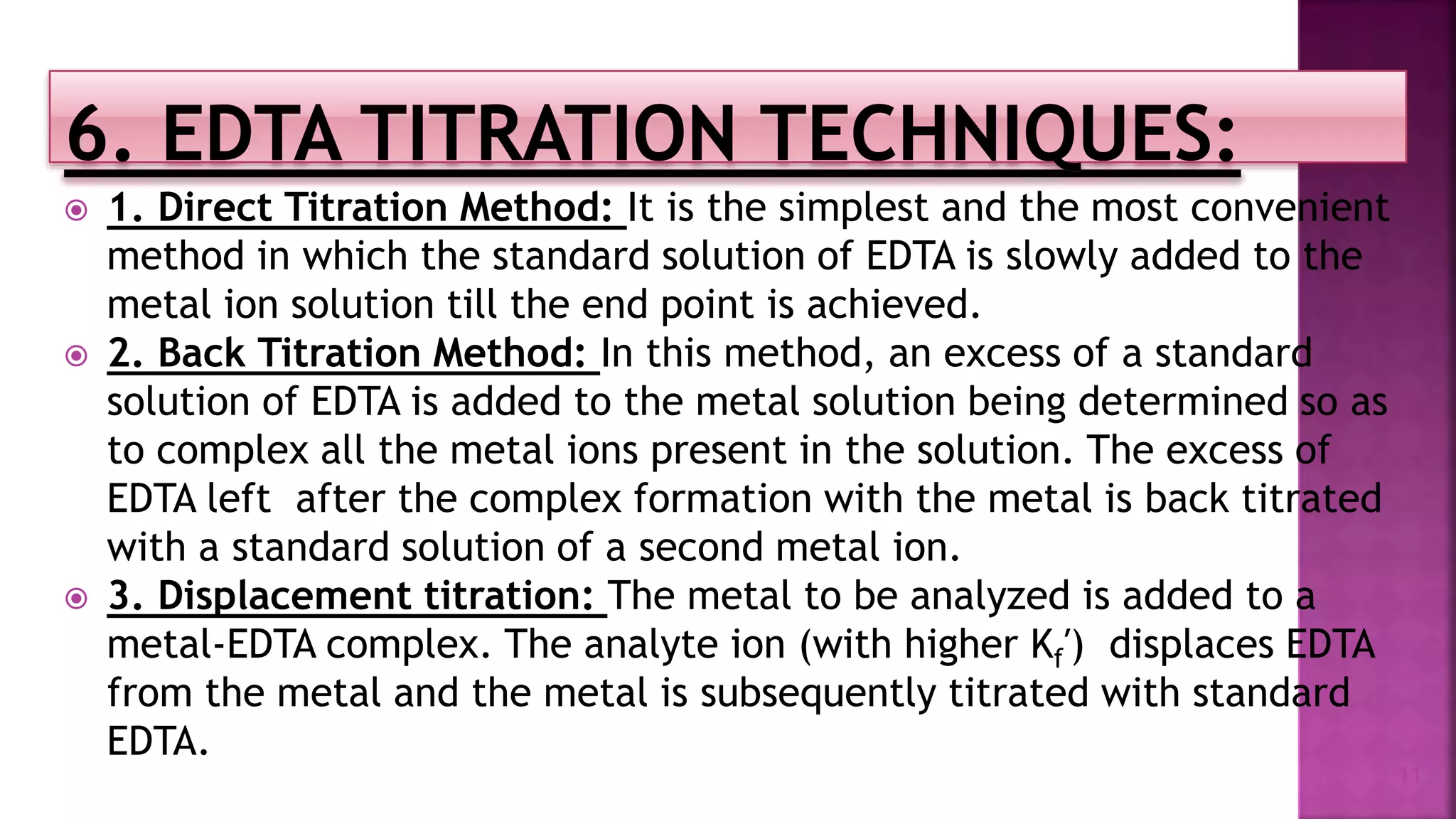
The document discusses EDTA titration, focusing on complexometric titration techniques for determining the concentration of metal ions. It covers various aspects, including types of ligands, chelating agents, indicators, and methods such as direct, back, displacement, and indirect titration. The use of buffering agents and masking/demasking agents is also highlighted for selective analysis of metal ion mixtures.





![ Mg ion complex with EDTA:
1. Mg2+ + In2- [MgIn]
(red colour)
2. [MgIn] + H4Y [MgY]2- + 2H+ + In2-
(blue)
15](https://image.slidesharecdn.com/complexometrictitration-170223173233/75/Complexometric-Titration-15-2048.jpg)
