Chemical kinetics is the study of reaction rates and mechanisms. The rate of a reaction describes how quickly reactants are converted to products and is affected by factors like concentration, temperature, catalysts, and surface area. The rate law expresses the reaction rate in terms of reactant concentrations and can be used to determine the order of a reaction. Integrated rate laws relate concentration over time and are used to calculate quantities like half-life, the time for half the reactants to be consumed.
























![The Rate Law
The rate law expresses the reaction rate as a function of reactant
concentrations, product concentrations, and temperature. It is
experimentally determined.
The derived rate law for a reaction must be consistent
with the postulated chemical mechanism of the reaction!
For a general reaction: aA + bB + ..... cC + dD
rate law: rate = k[A]m
[B]n
........
k = rate constant
m and n = reaction orders (not related to a, b,...)
(for a unidirectional (one-way) reaction)!](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-25-2048.jpg)
![Rate law : R = k [A]
α
[B]
β
...
Rate Constant
Reaction order
• The rate of reaction is often found to be proportional to the
molar concentrations of the reactants raised to a simple power.
• It cannot be overemphasized that reaction orders have no
relation to stoichiometric coefficients, and they are determined by
experiment.](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-26-2048.jpg)
![Order of the reaction
Order of a reaction is defined as the sum of the exponential powers to which each
concentration is raised in the rate expression.
For example, if the overall rate is given by the expression
Rate = k [A]m
[B]n
Then, the overall order of the reaction is (m+n). The order with respect to A is m.
The order with respect to B is n. If m=1; n=0 and vice versa, the order of the
reaction is 1, and the reaction is called first order. If m=1, n=1, the order of the
reaction is 2 and the reaction is called second order and so on. A zero order
reaction is one where the reaction rate does not depend upon the concentration of
the reactant. In this type of reaction, the rate constant is equal to the rate of the
reaction.](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-27-2048.jpg)

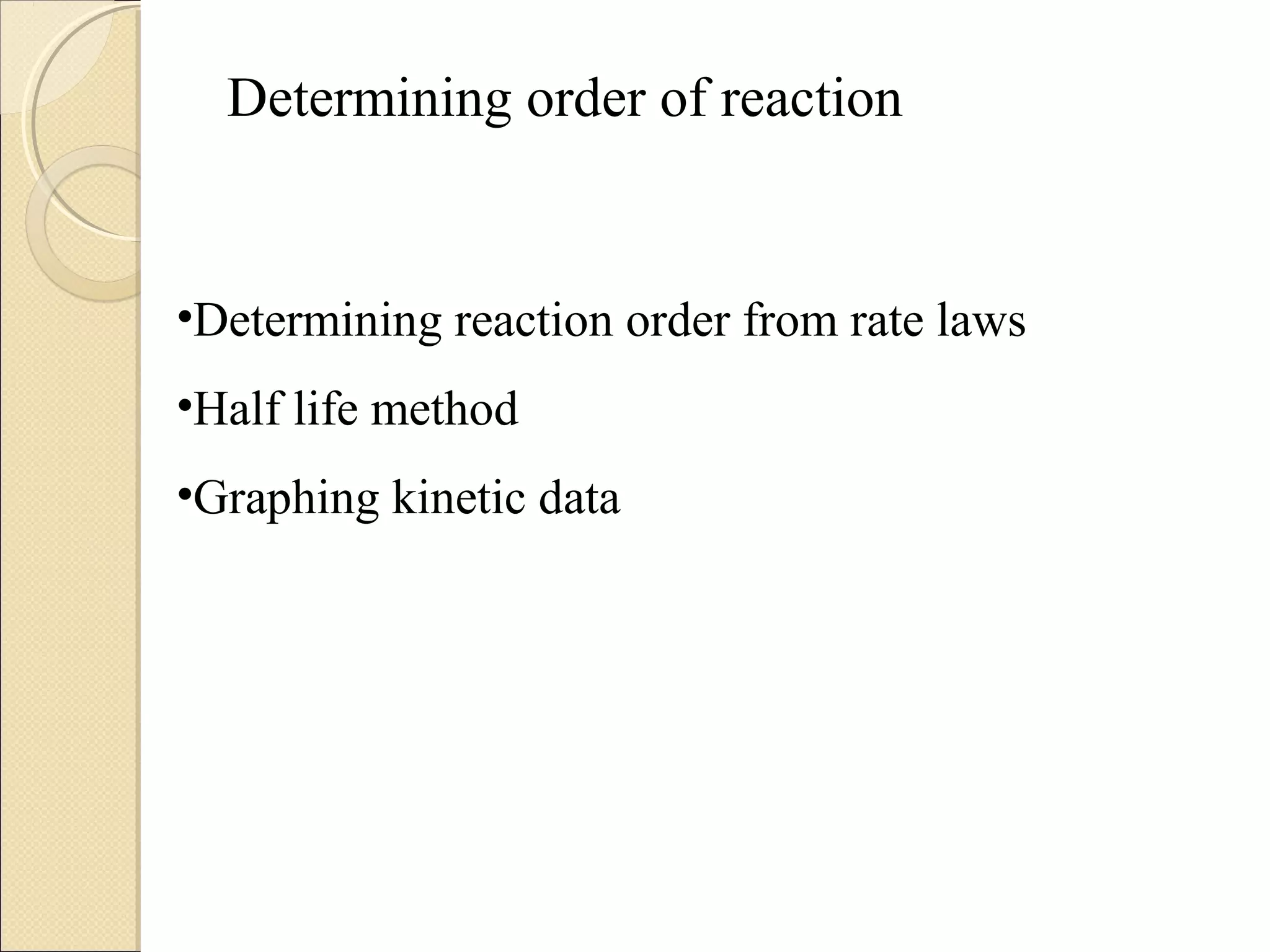
![Concentration-Time EquationsConcentration-Time Equations
First-Order Integrated Rate Law
– You could write the rate law in the form
]A[k
t
]A[
Rate =
∆
∆
−=](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-30-2048.jpg)
![Concentration-Time EquationsConcentration-Time Equations
First-Order Integrated Rate Law
– Using calculus, you get the following equation.
kt-
]A[
]A[
ln
o
t
=
– Here [A]t is the concentration of reactant A at time t, and [A]o is the
initial concentration.
– The ratio [A]t/[A]o is the fraction of A remaining at time t.](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-31-2048.jpg)
![Concentration-Time EquationsConcentration-Time Equations
Second-Order Integrated Rate Law
– You could write the rate law in the form
2
]A[k
t
]A[
Rate =
∆
∆
−=](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-32-2048.jpg)
![Concentration-Time EquationsConcentration-Time Equations
Second-Order Integrated Rate Law
– Using calculus, you get the following equation.
ot [A]
1
kt
]A[
1
+=
– Here [A]t is the concentration of reactant A
at time t, and [A]o is the initial concentration.](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-33-2048.jpg)
![Concentration-Time EquationsConcentration-Time Equations
Zero-Order Integrated Rate Law
– The Zero-Order Integrated Rate Law equation
is:.
o]A[kt]A[ +−=](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-34-2048.jpg)




![Half-lifeHalf-life
For a second-order reaction, half-life
depends on the initial concentration and
becomes larger as time goes on.
– Again, assuming that [A]t = ½[A]o after one half-life, it can be shown that:
o]A[k
1
t2
1 =](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-39-2048.jpg)
![Half-lifeHalf-life
For Zero-Order reactions, the half-lite is
dependent upon the initial concentration
of the reactant and becomes shorter as
the reaction proceeds.
k2
]A[
t o
2
1 =](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-40-2048.jpg)

![Graphing Kinetic DataGraphing Kinetic Data
In addition to the method of initial rates, rate laws can
be deduced by graphical methods.
– If we rewrite the first-order concentration-
time equation in a slightly different form, it
can be identified as the equation of a straight
line.
ot ]Aln[kt]Aln[ +−=
y = mx + b
This means if you plot ln[A] versus time, you will get a straight line for a
first-order reaction.](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-42-2048.jpg)
![Graphing Kinetic DataGraphing Kinetic Data
– If we rewrite the second-order concentration-time equation in a slightly
different form, it can be identified as the equation of a straight line.
ot ]A[
1
kt
]A[
1
+=
y = mx + b
This means if you plot 1/[A] versus time, you will get a straight line
for a second-order reaction.](https://image.slidesharecdn.com/kineticsmain-170911175307/75/Kinetic-for-Pharmaceutical-analysis-and-Physical-Pharmacy-43-2048.jpg)




















