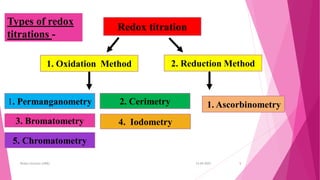
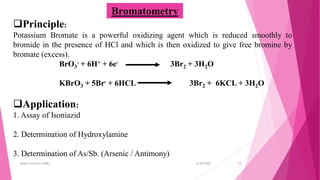
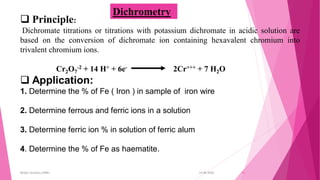
This document discusses redox titrations. It begins by defining oxidation and reduction reactions. It then discusses different types of redox titrations including cerimetry, iodimetry, iodometry, bromatometry, dichrometry, and titration with potassium iodate. For each type of titration, the document describes the basic principles and provides some examples of applications. The document is presented by Miss Harshada R. Bafna and contains information on concepts, types, and specific techniques for various redox titration methods.