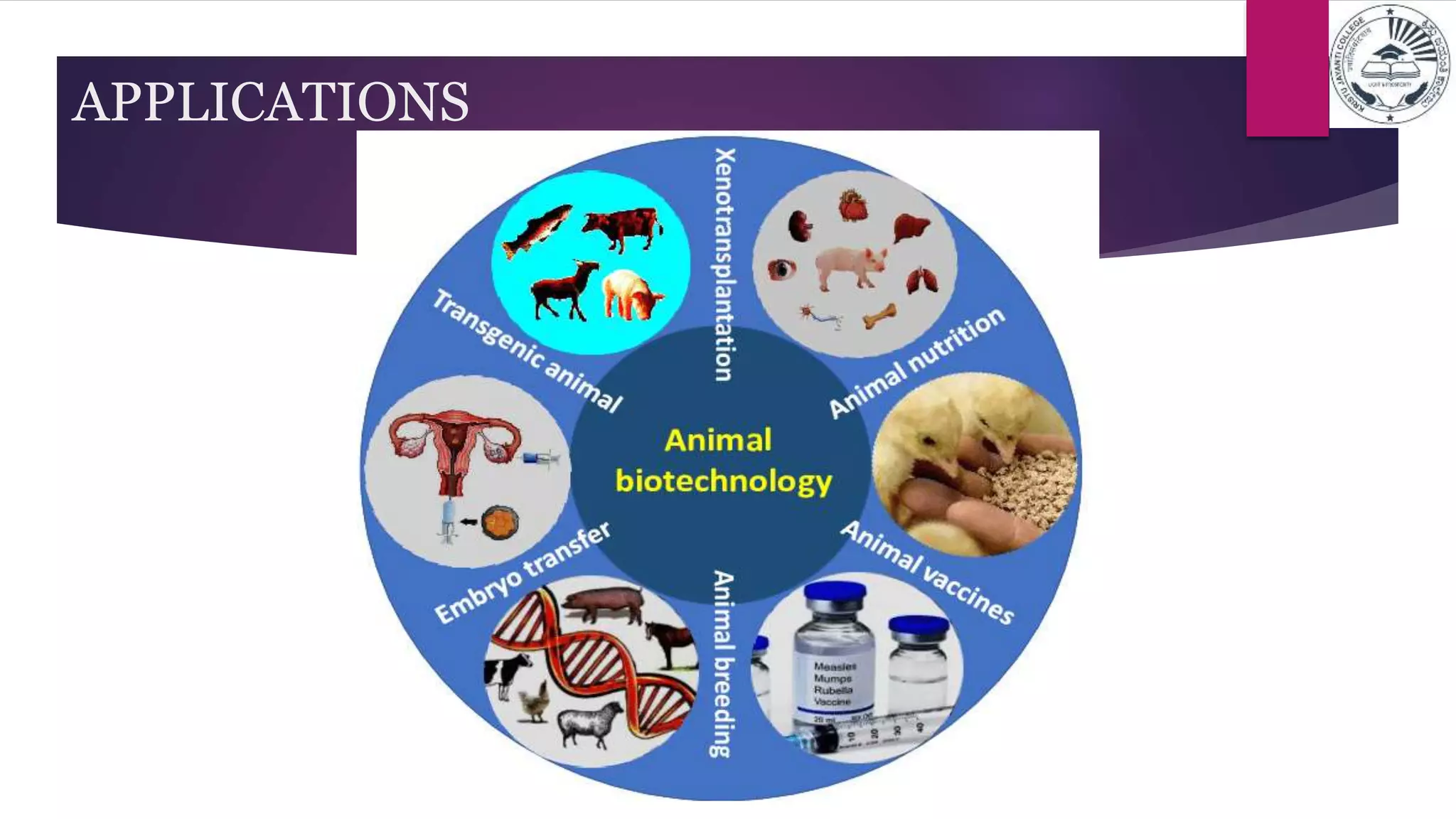
This document provides information about animal biotechnology through several sections. It begins with an introduction that discusses the long history of animal biotechnology including traditional breeding techniques dating back to 5000 BC. It then covers the history of animal biotechnology from the 1970s to the present day, highlighting important milestones. Several sections follow on the scope, applications, and terminology of animal biotechnology including transgenic animals, cloning, animal models in research, vaccines, nutrition, and embryo transfer. The document concludes by defining common terminology used in animal cell culture.