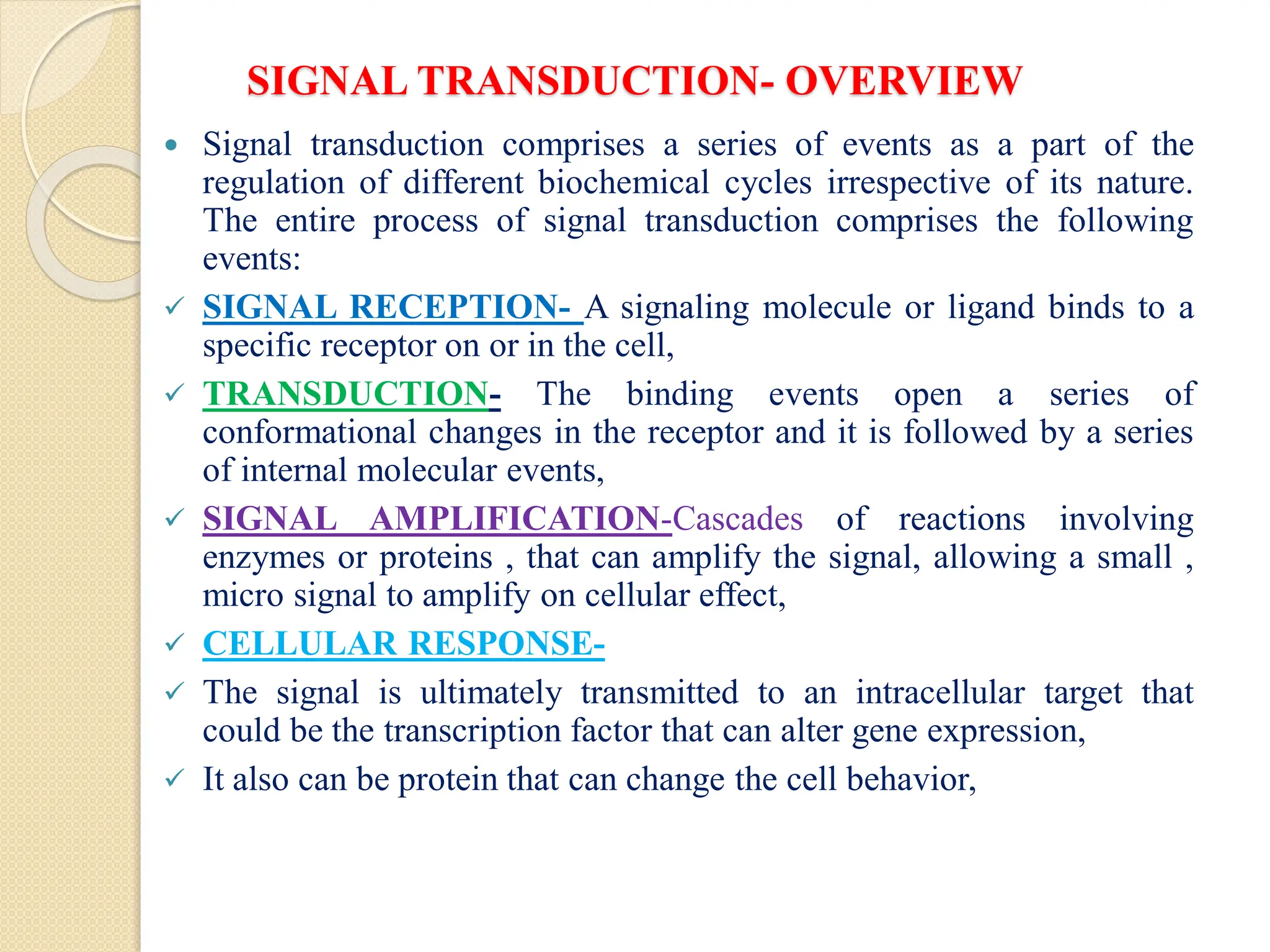
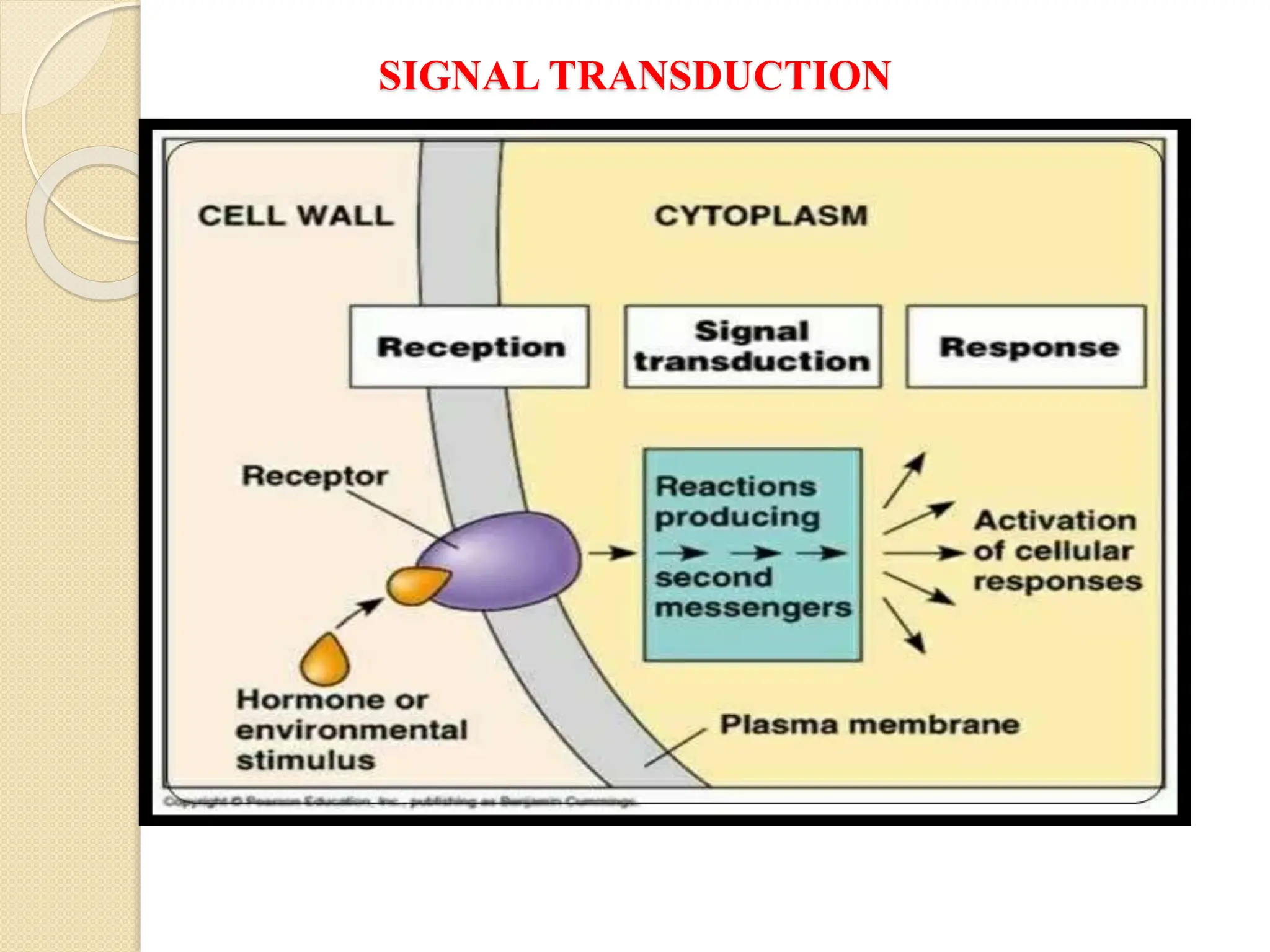
The resource provides a very brief idea about the inbuilt system of cellular communication and its related messengers while used to cell talks to the adjoining cells and other cells from the far off the different body parts. The signal transduction pathway has been widely covered to explore the molecular magic of cell talk in the context of the regulation of biochemical pathways along with the homeostasis. It also the different supporting agents to mediate the entire process despite the number of issues arise in course of cell talking.









![GPCR- MODE OF FUNCTION
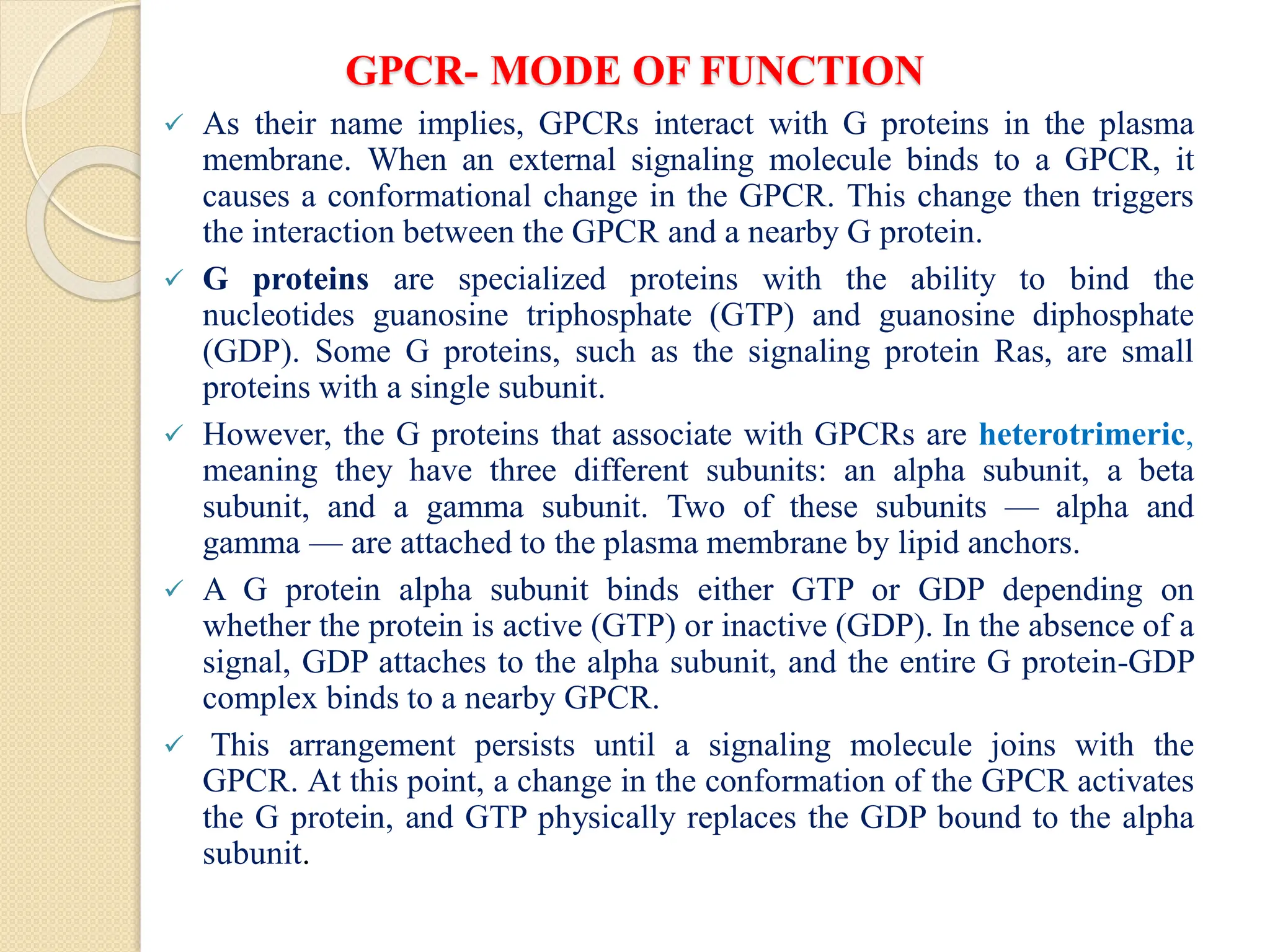
Vertebrate genomes contain multiple genes that encode the alpha, beta,
and gamma subunits of G proteins. The many different subunits
encoded by these genes combine in multiple ways to produce a diverse
family of G proteins.
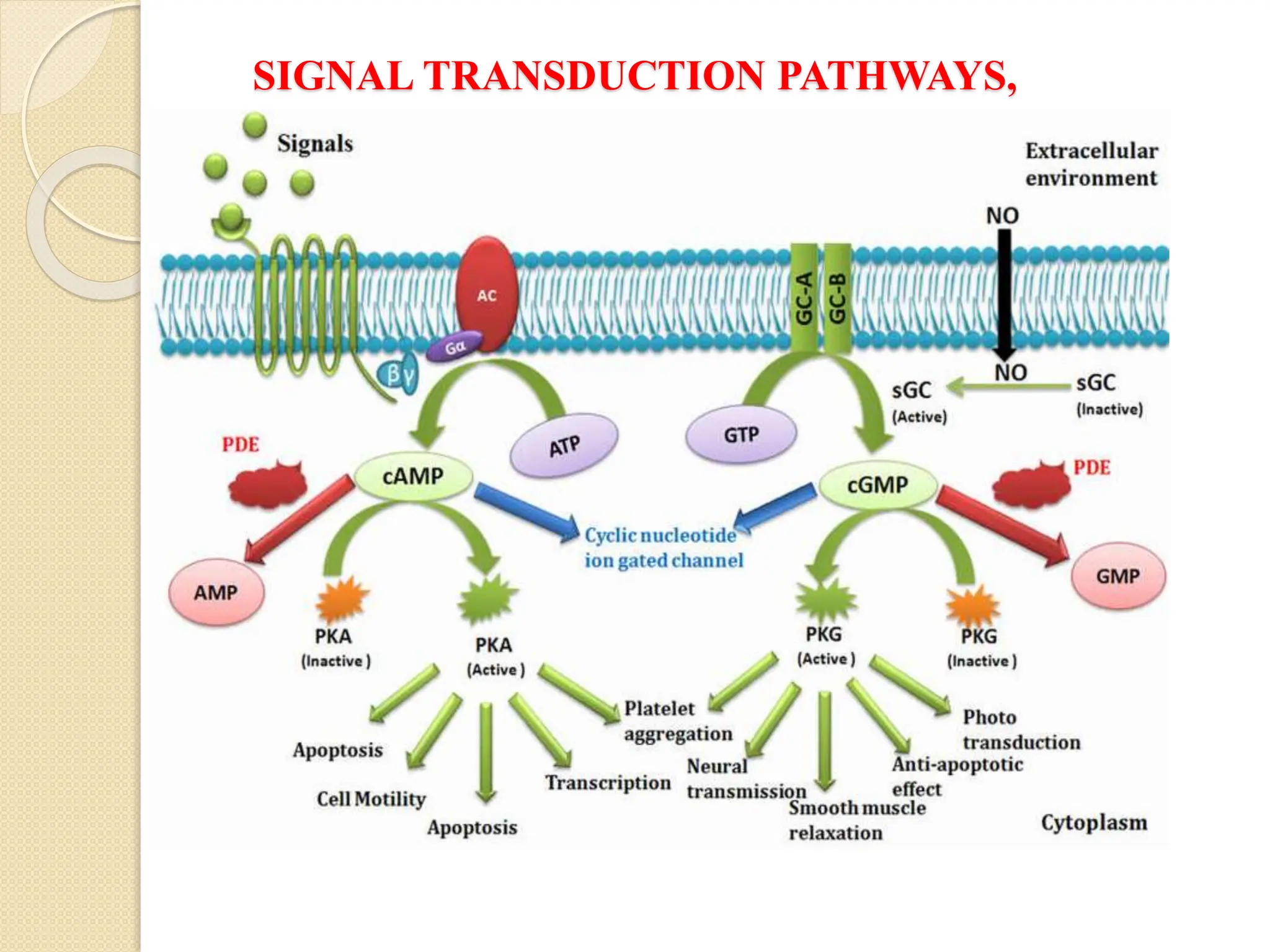
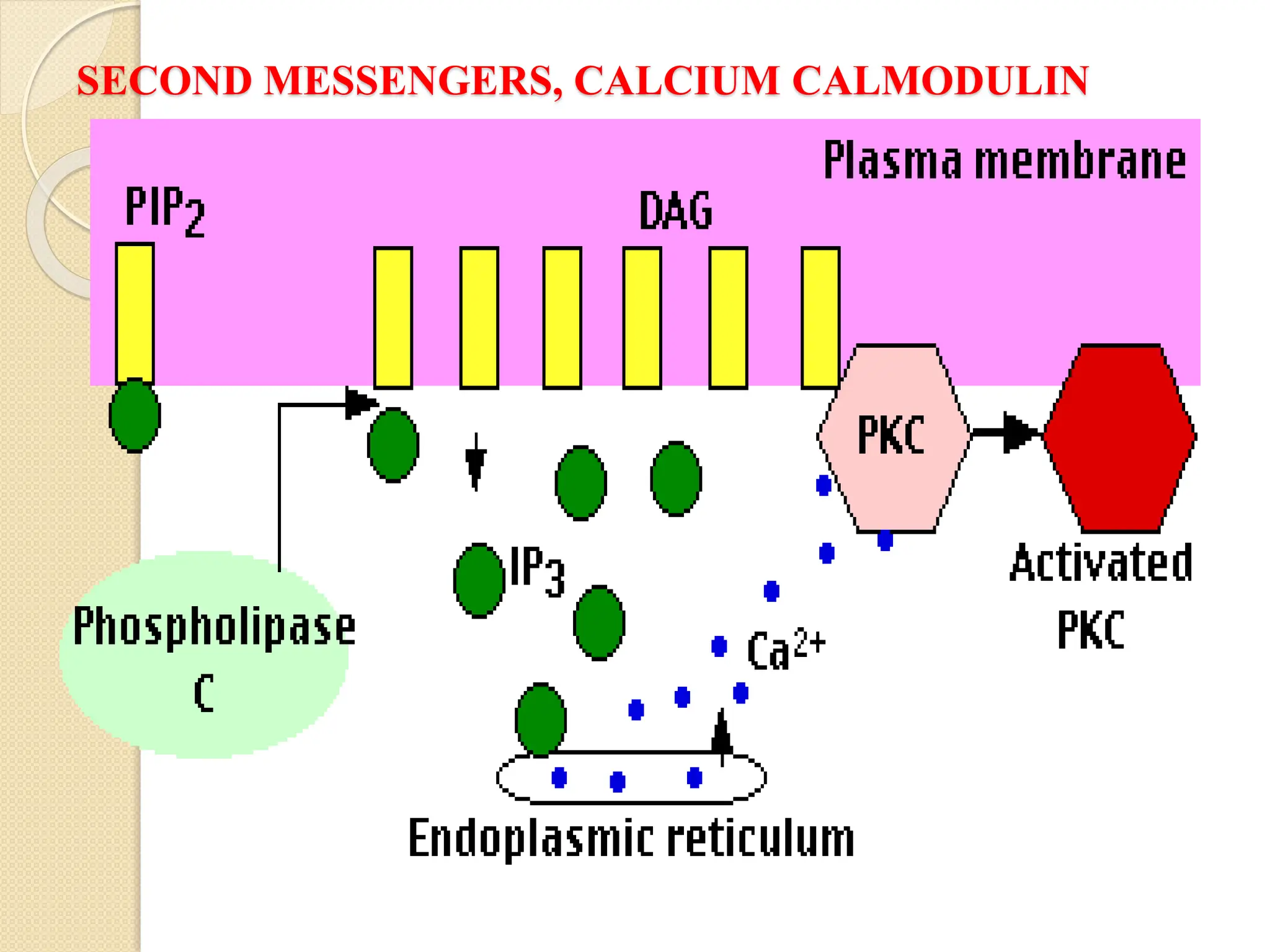
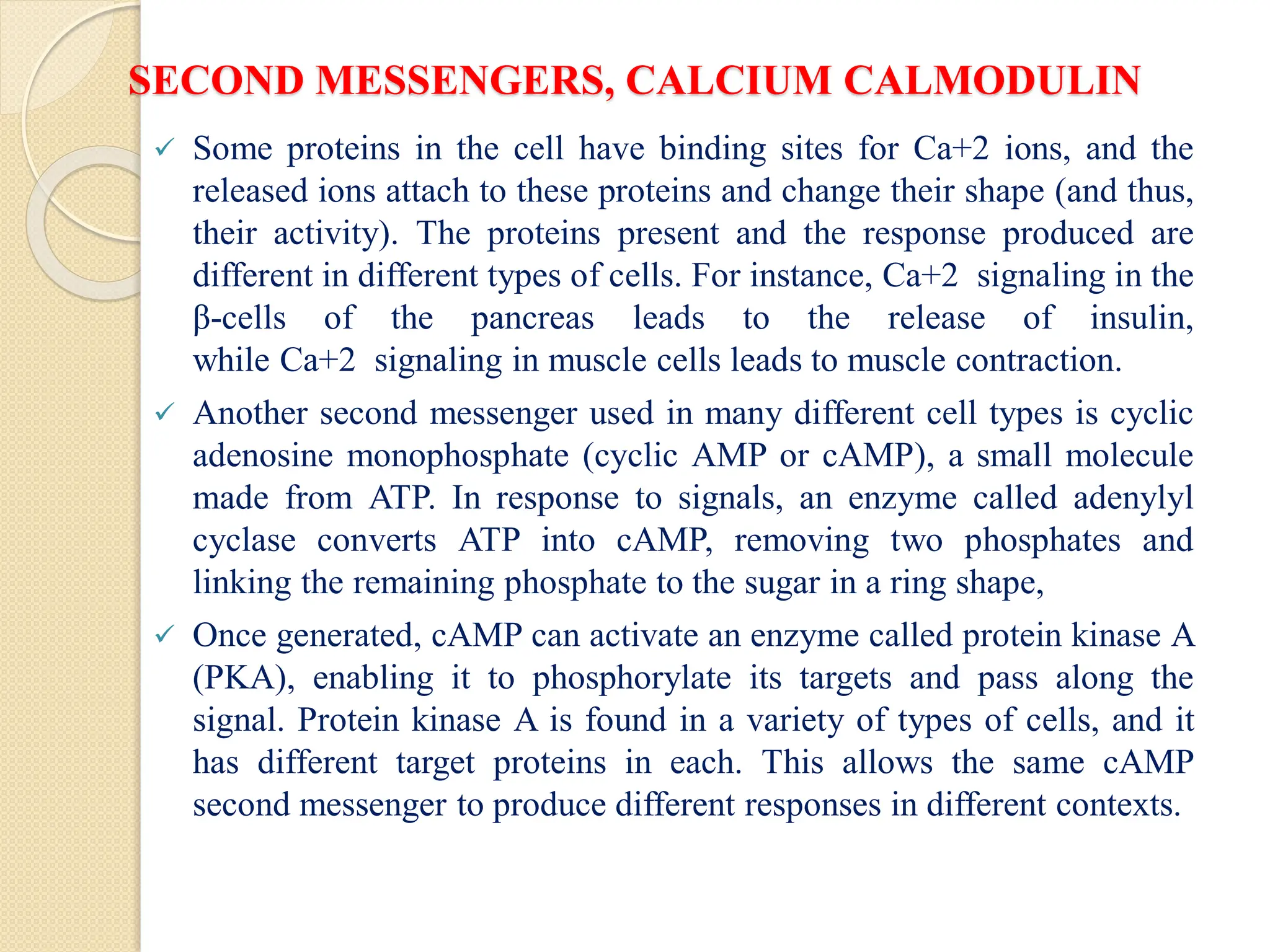
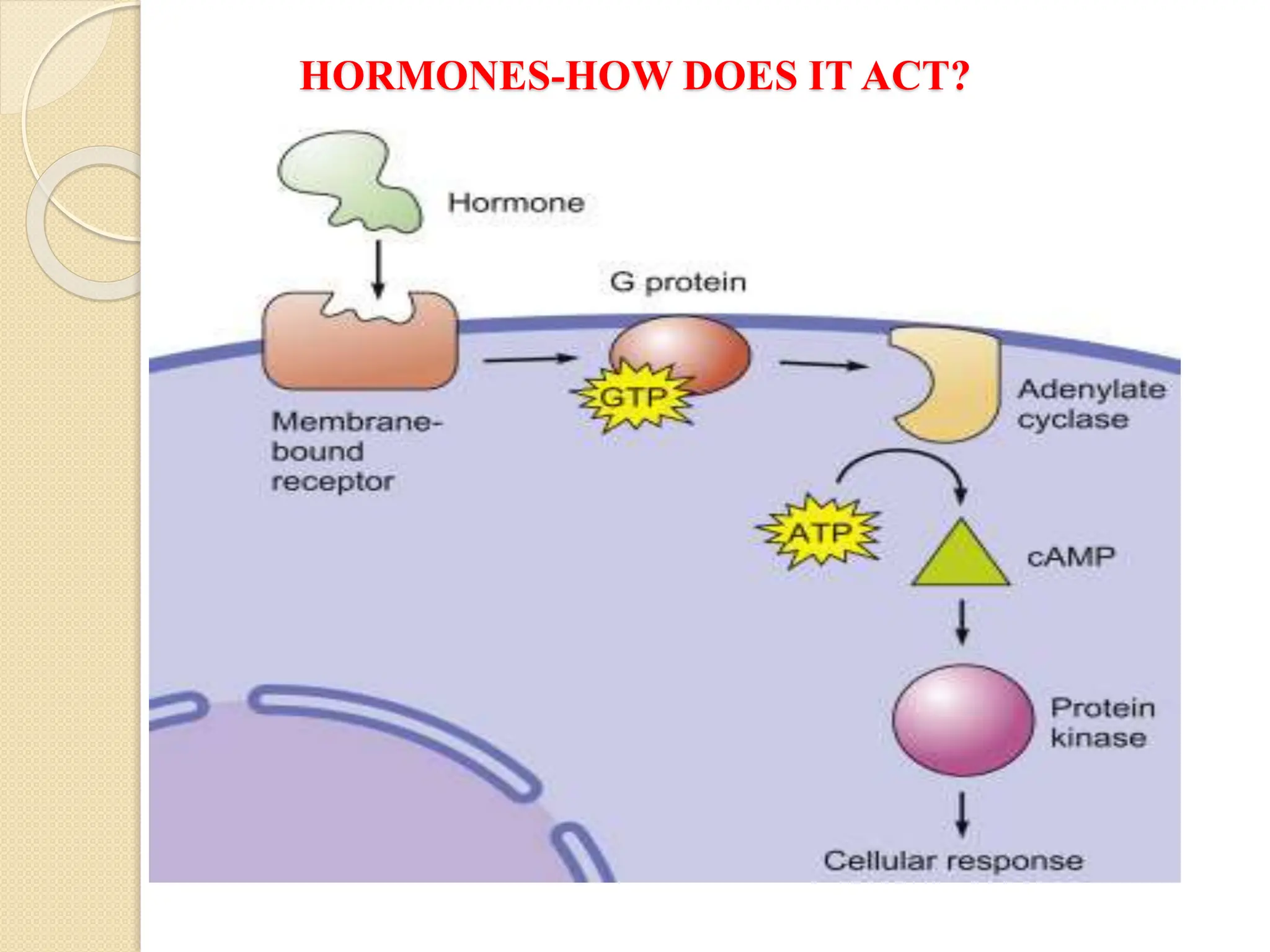
What Second Messengers Do GPCR Signals Trigger in Cells?
Activation of a single G protein can affect the production of hundreds
or even thousands of second messenger molecules. (Recall that second
messengers — such as cyclic AMP [cAMP], diacylglycerol [DAG],
and inositol 1, 4, 5-triphosphate [IP3] - are small molecules that
initiate and coordinate intracellular signaling pathways.)
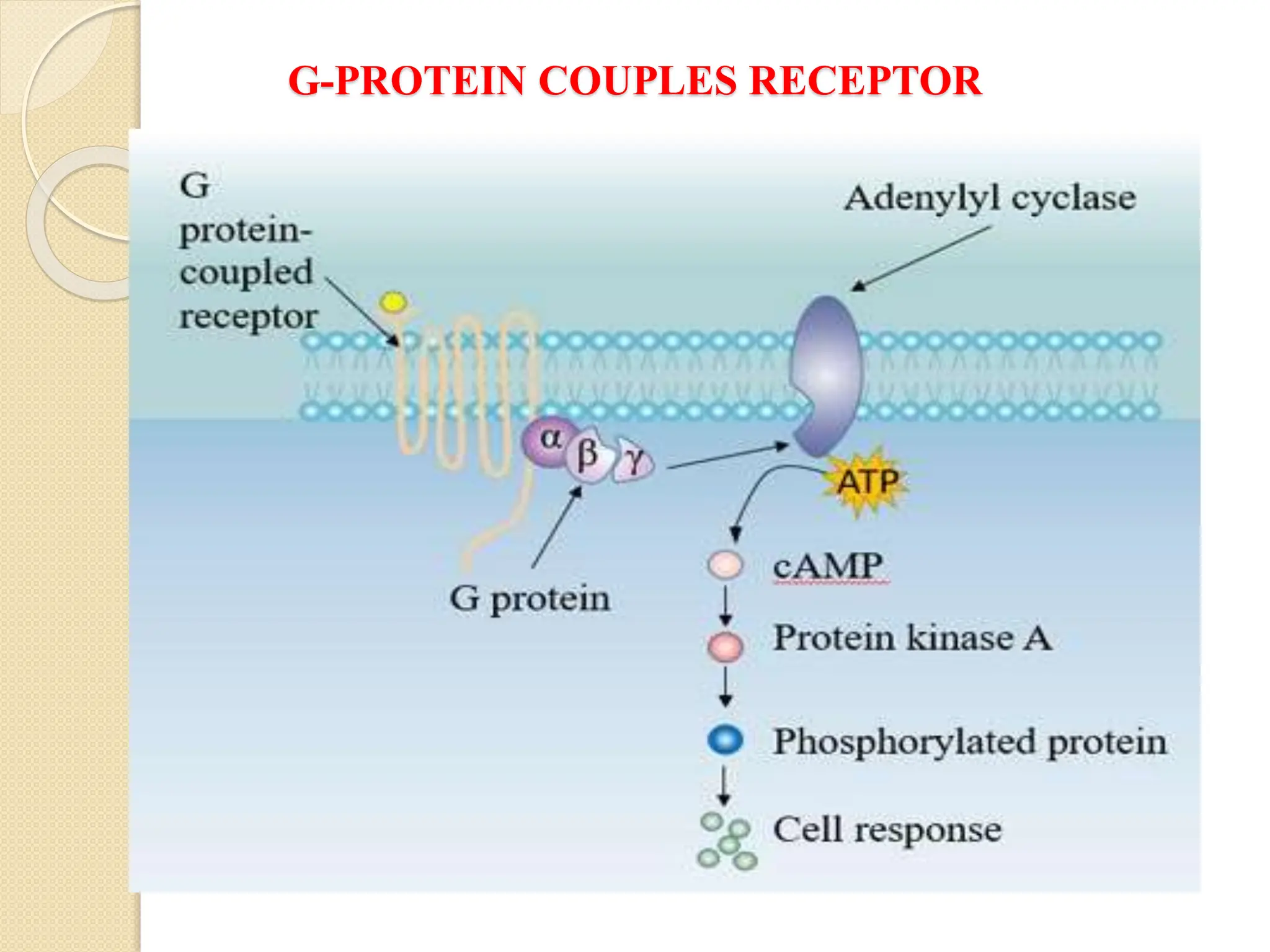
One especially common target of activated G proteins is adenylyl
cyclase, a membrane-associated enzyme that, when activated by the
GTP-bound alpha subunit, catalyzes synthesis of the second
messenger cAMP from molecules of ATP. In humans, cAMP is
involved in responses to sensory input, hormones, and nerve
transmission, among others.
Phospholipase C is another common target of activated G proteins](https://image.slidesharecdn.com/signaltransduction-251211120233-b2cda692/75/Cellular-Communication-Signal-Transduction-Pathway-20-2048.jpg)