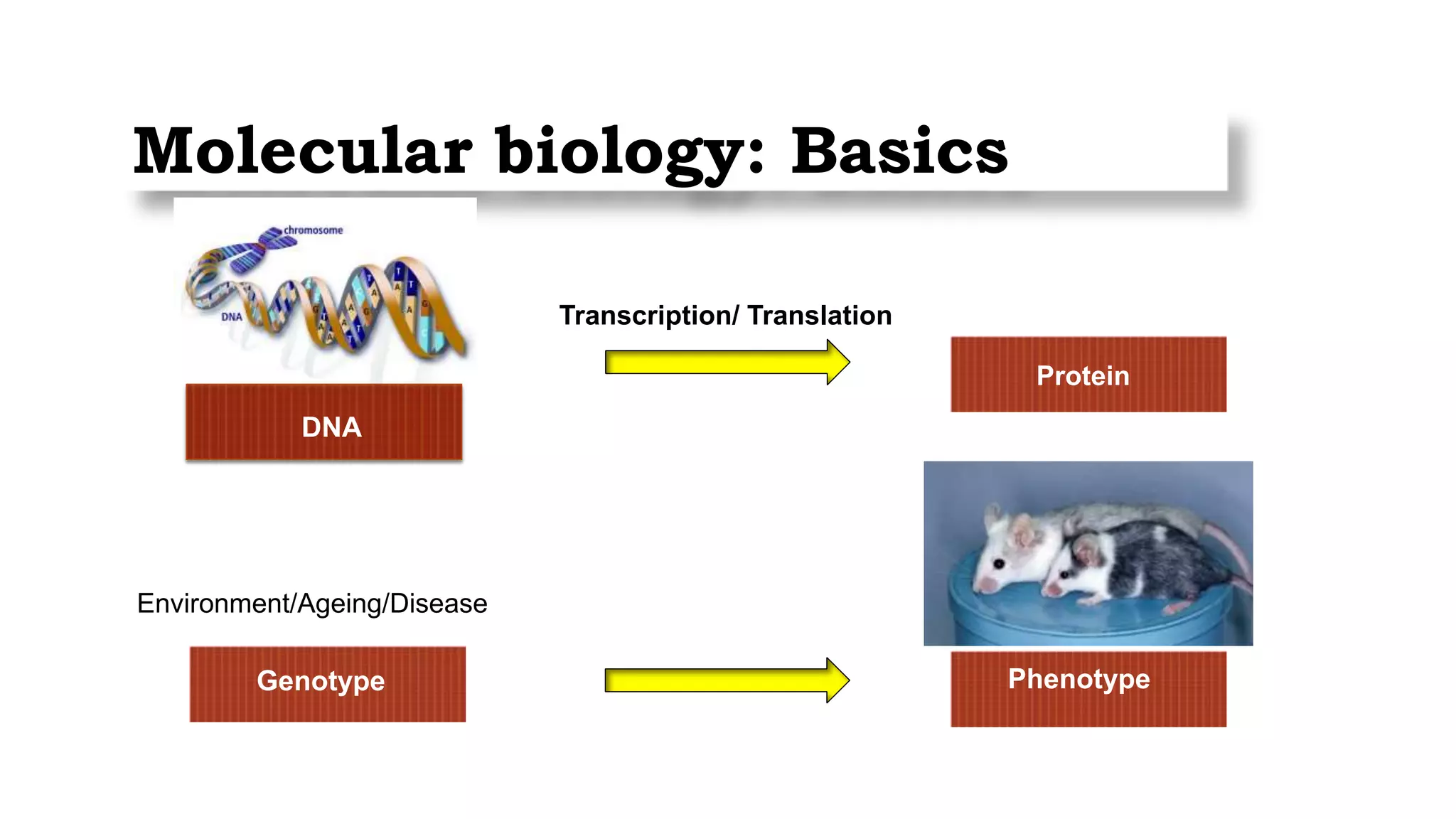
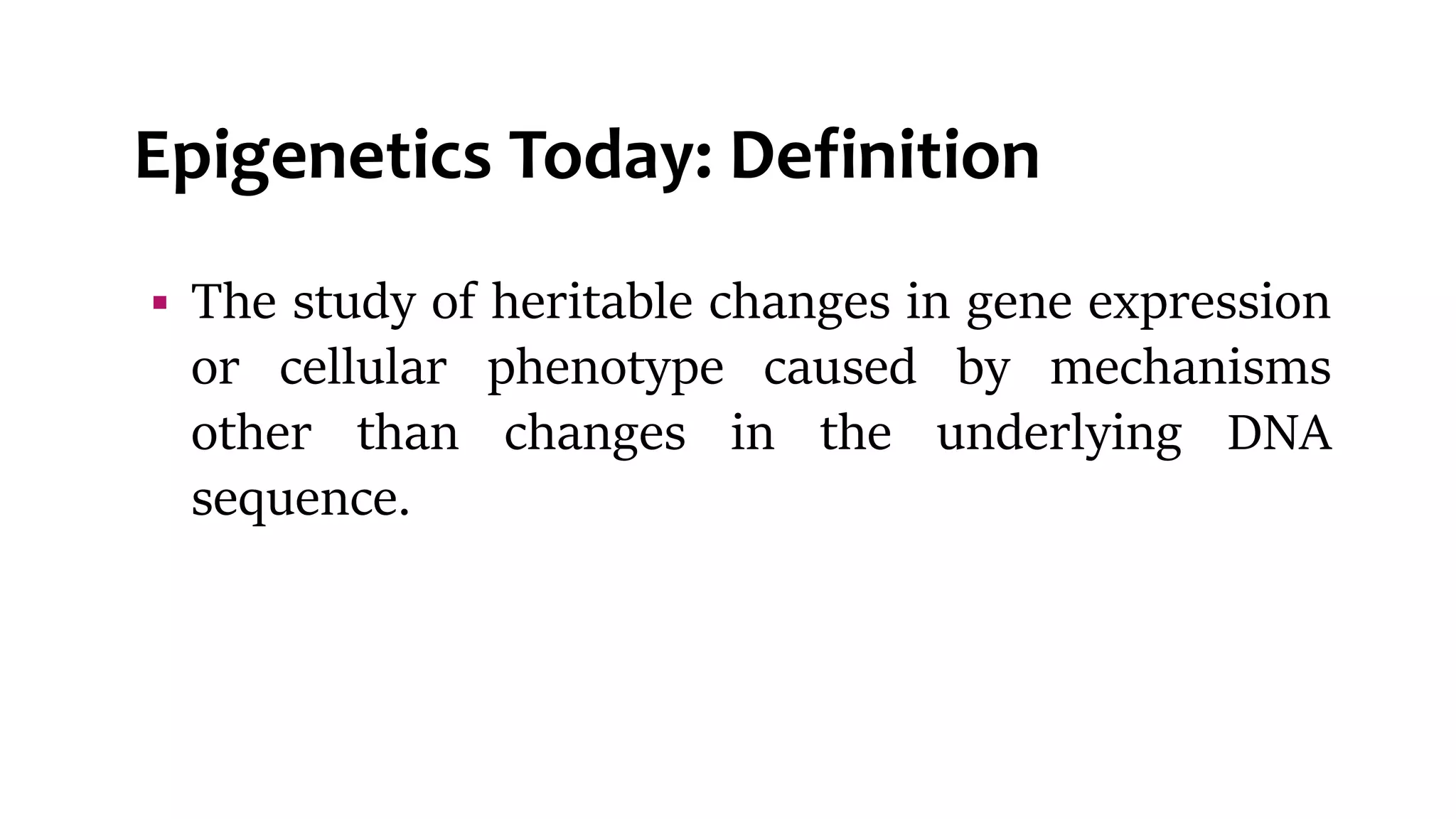
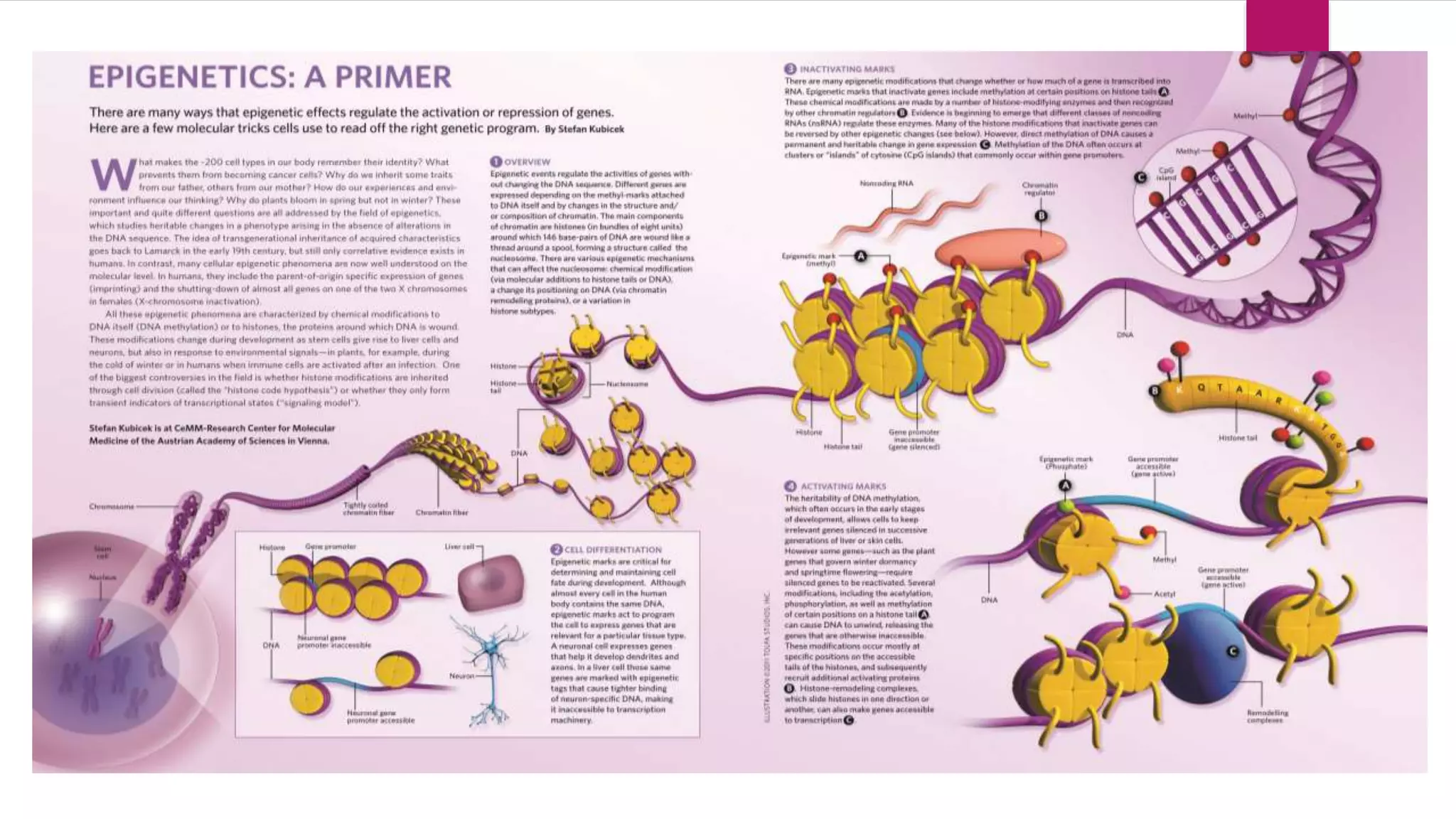
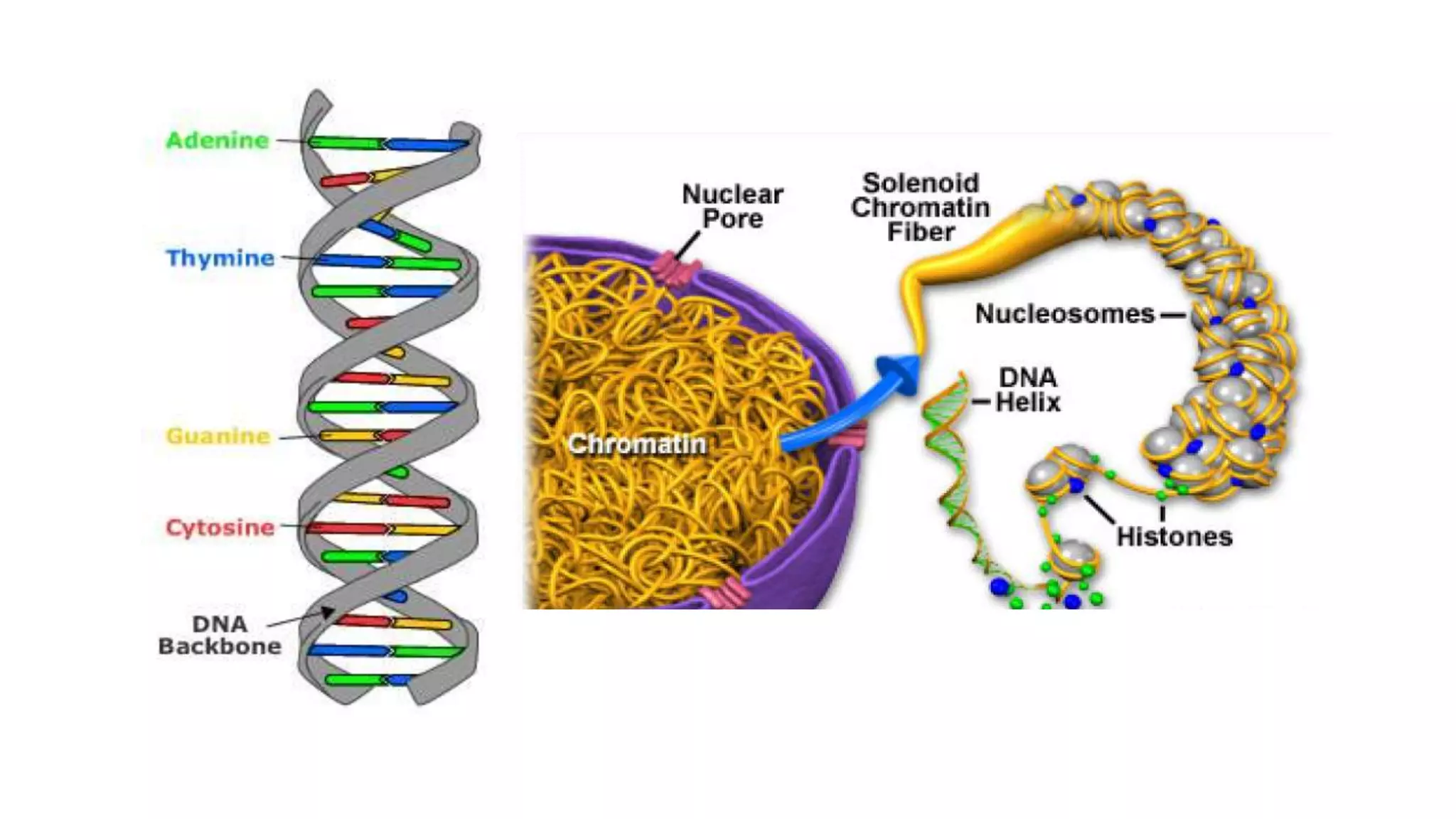
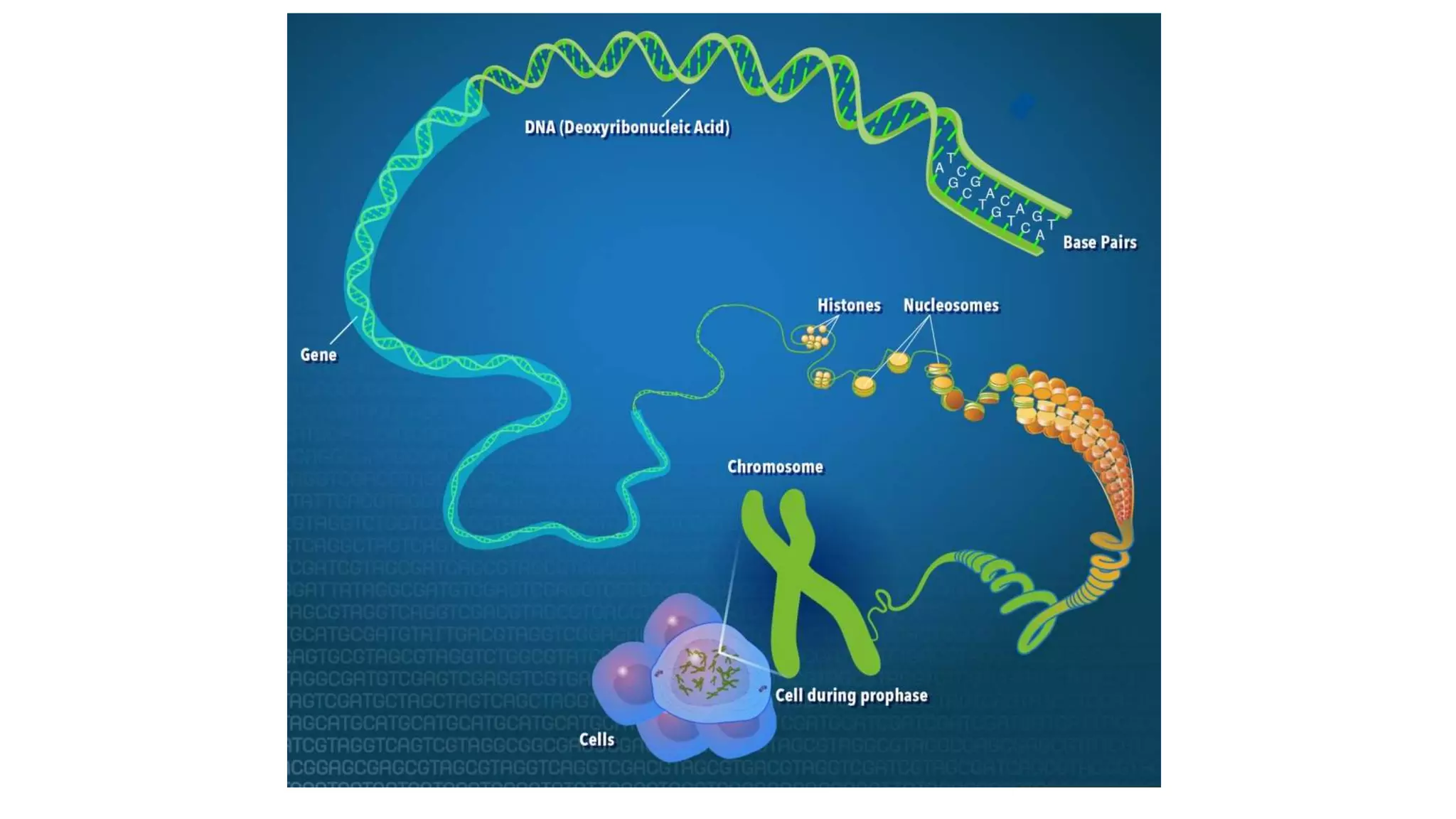
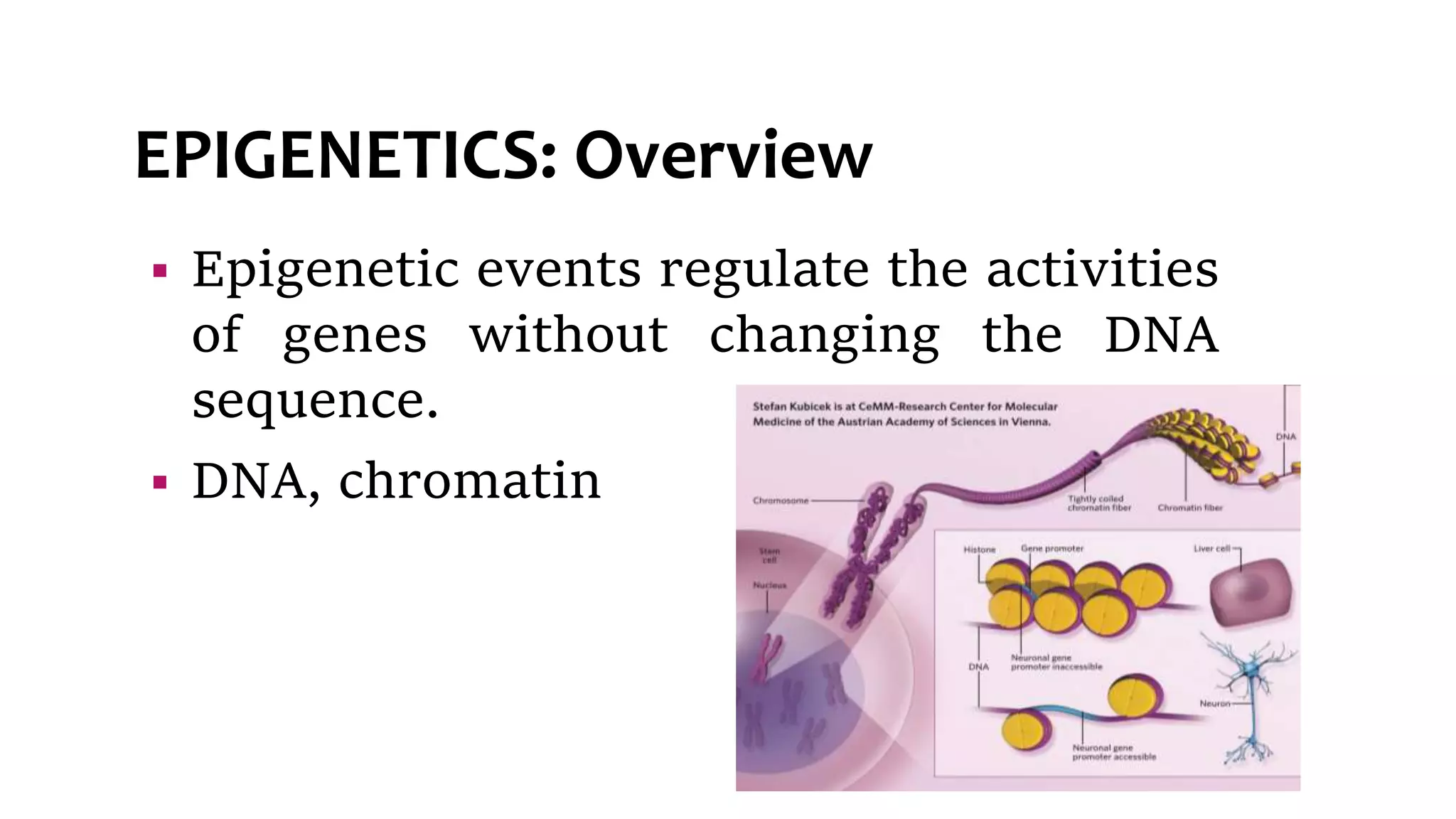
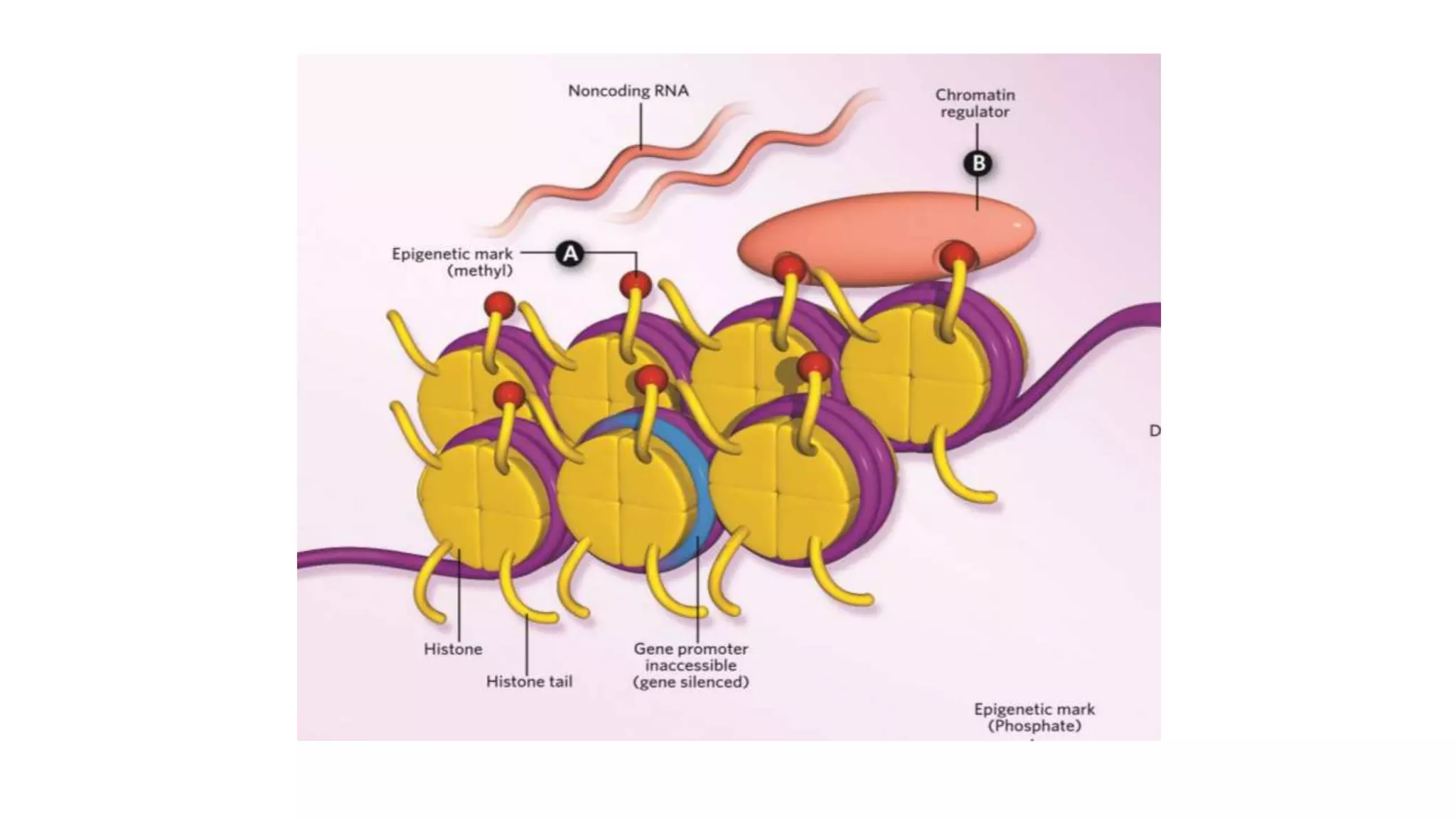
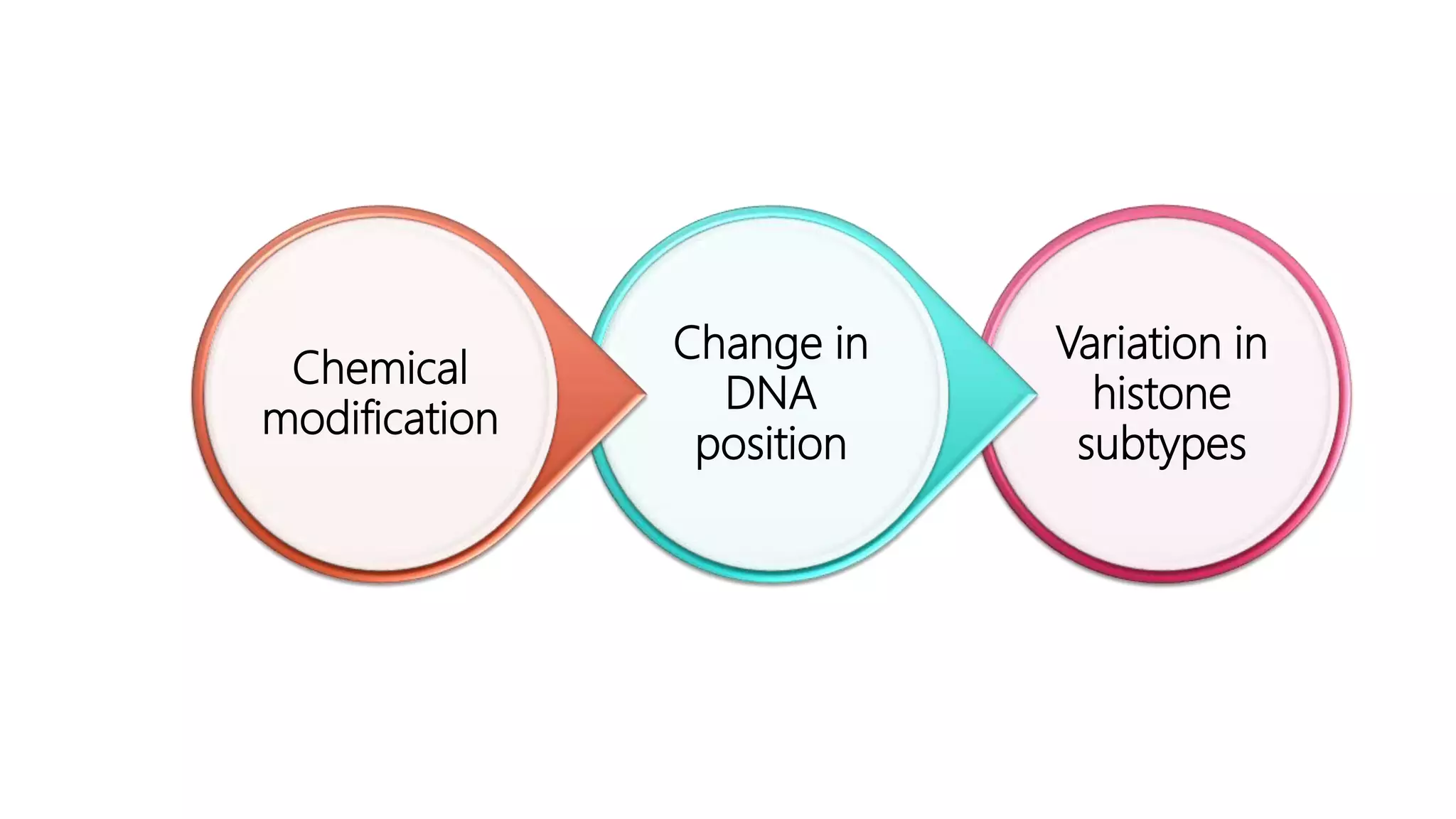
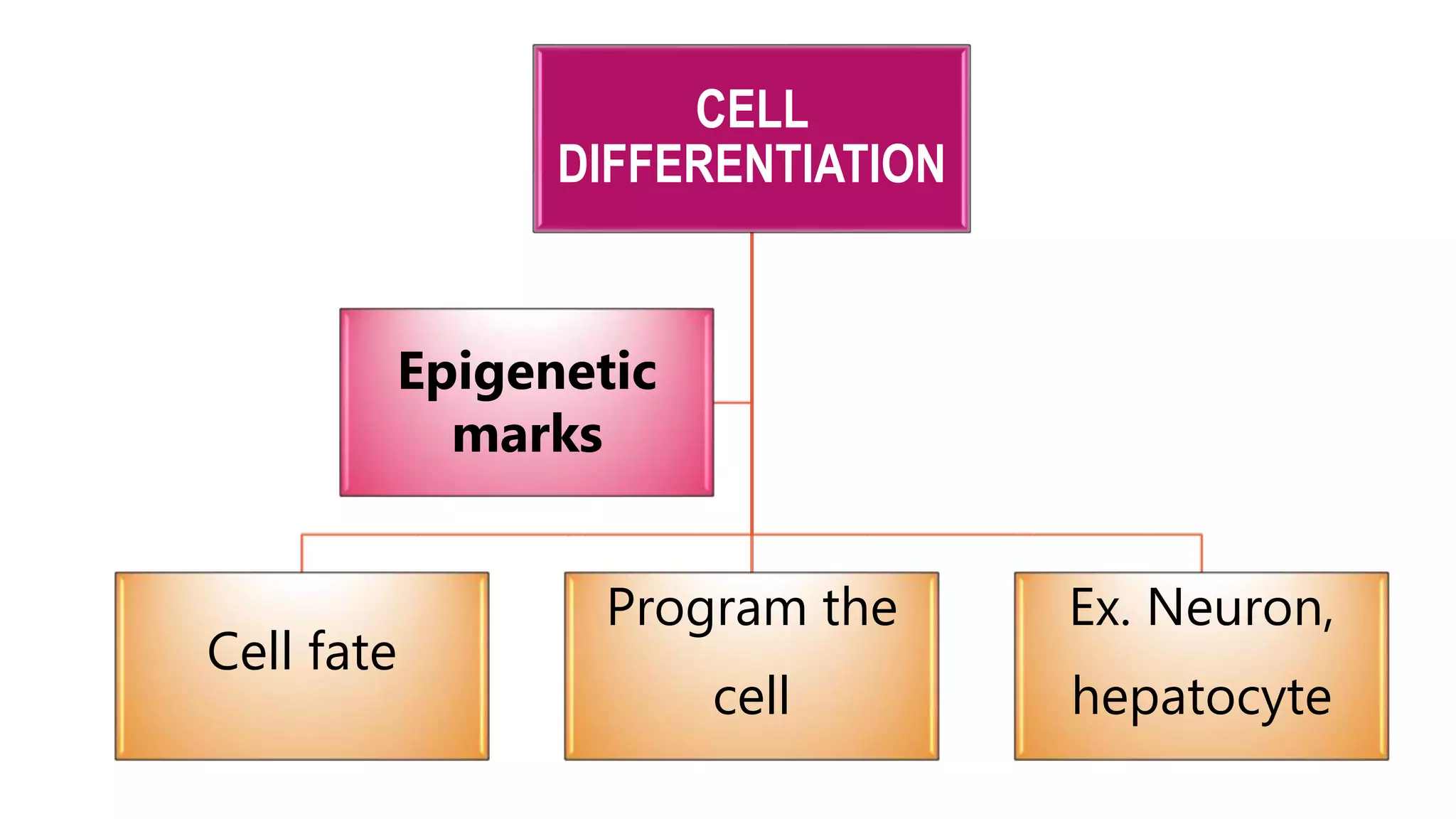
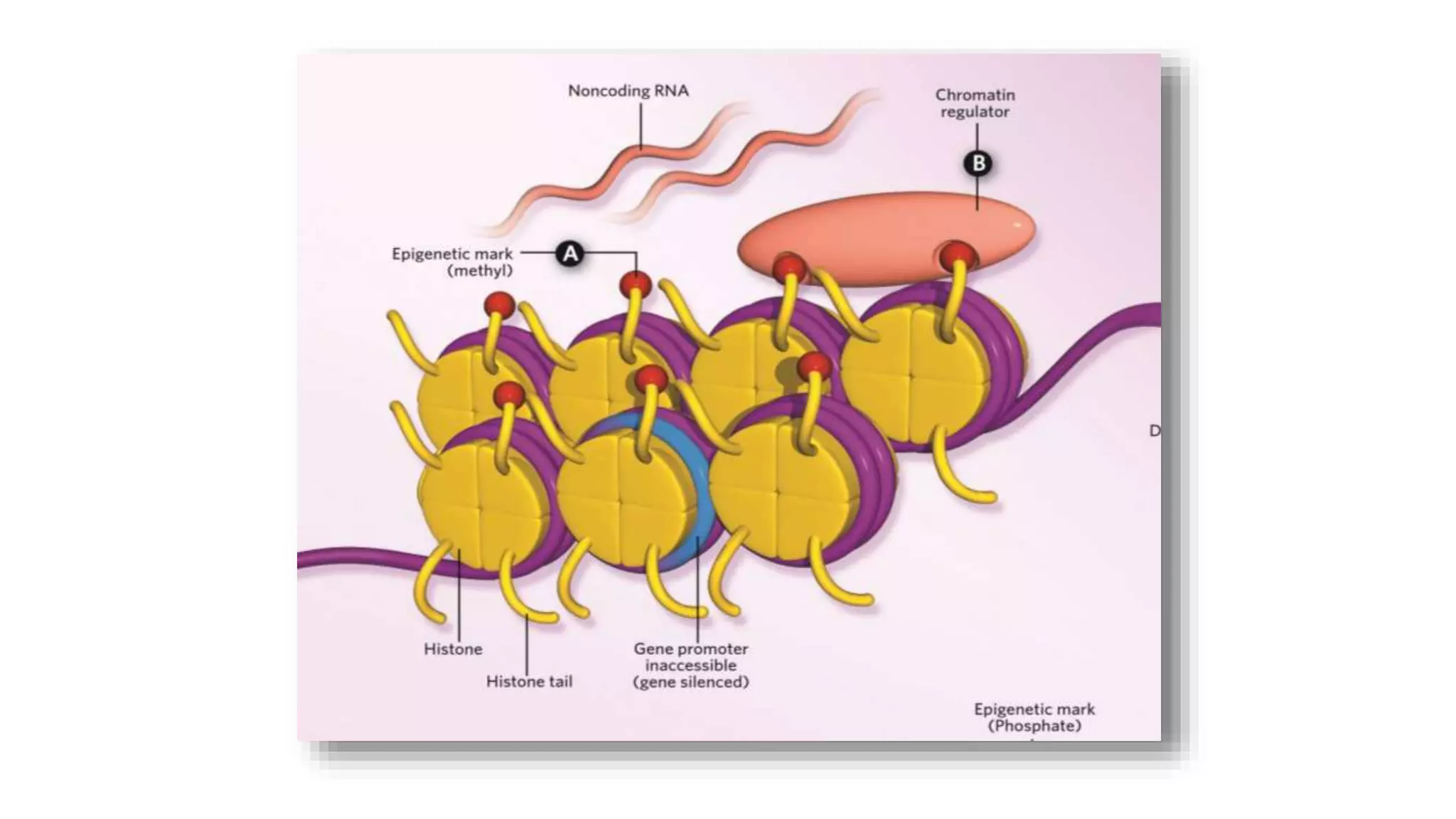
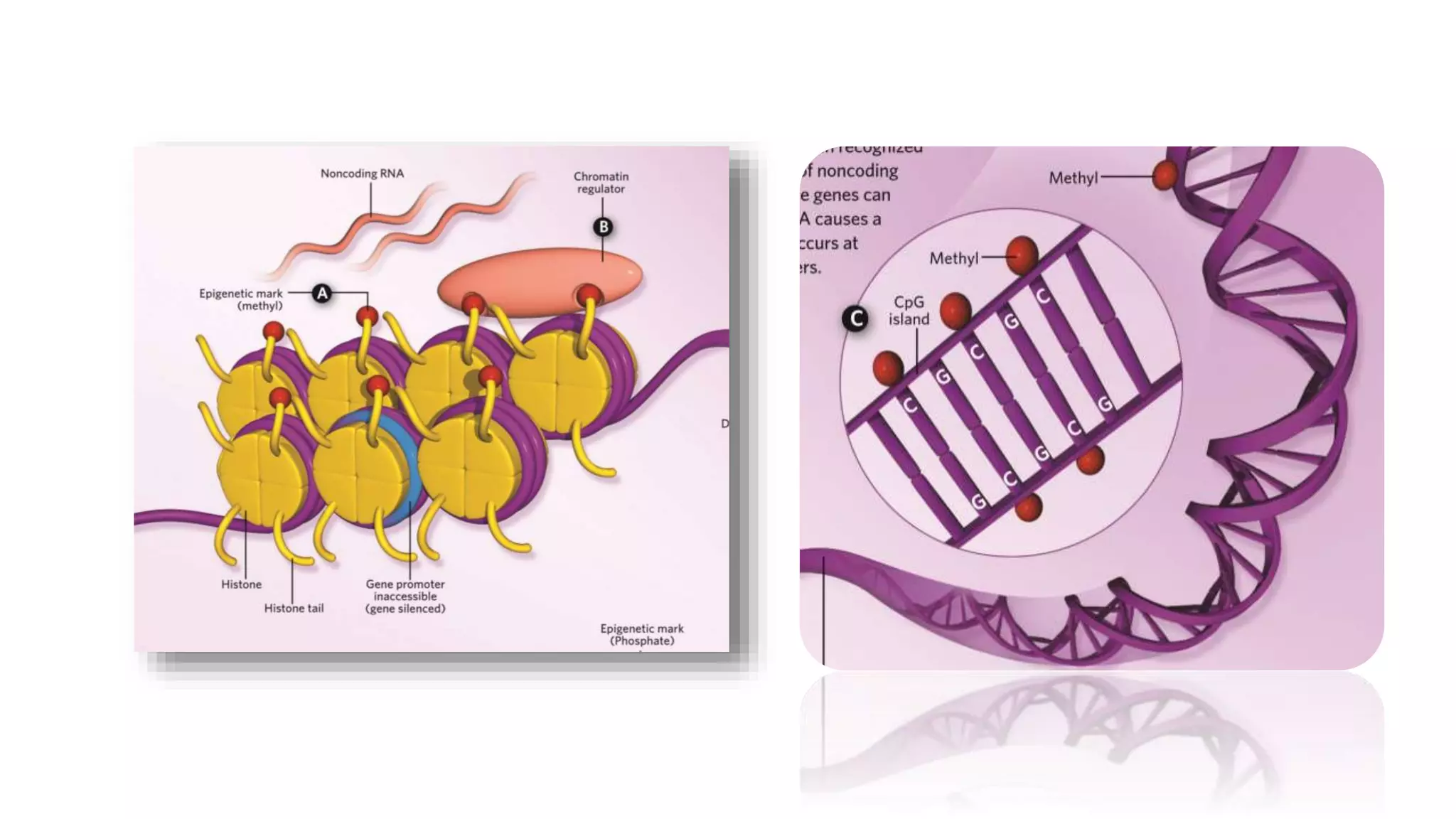
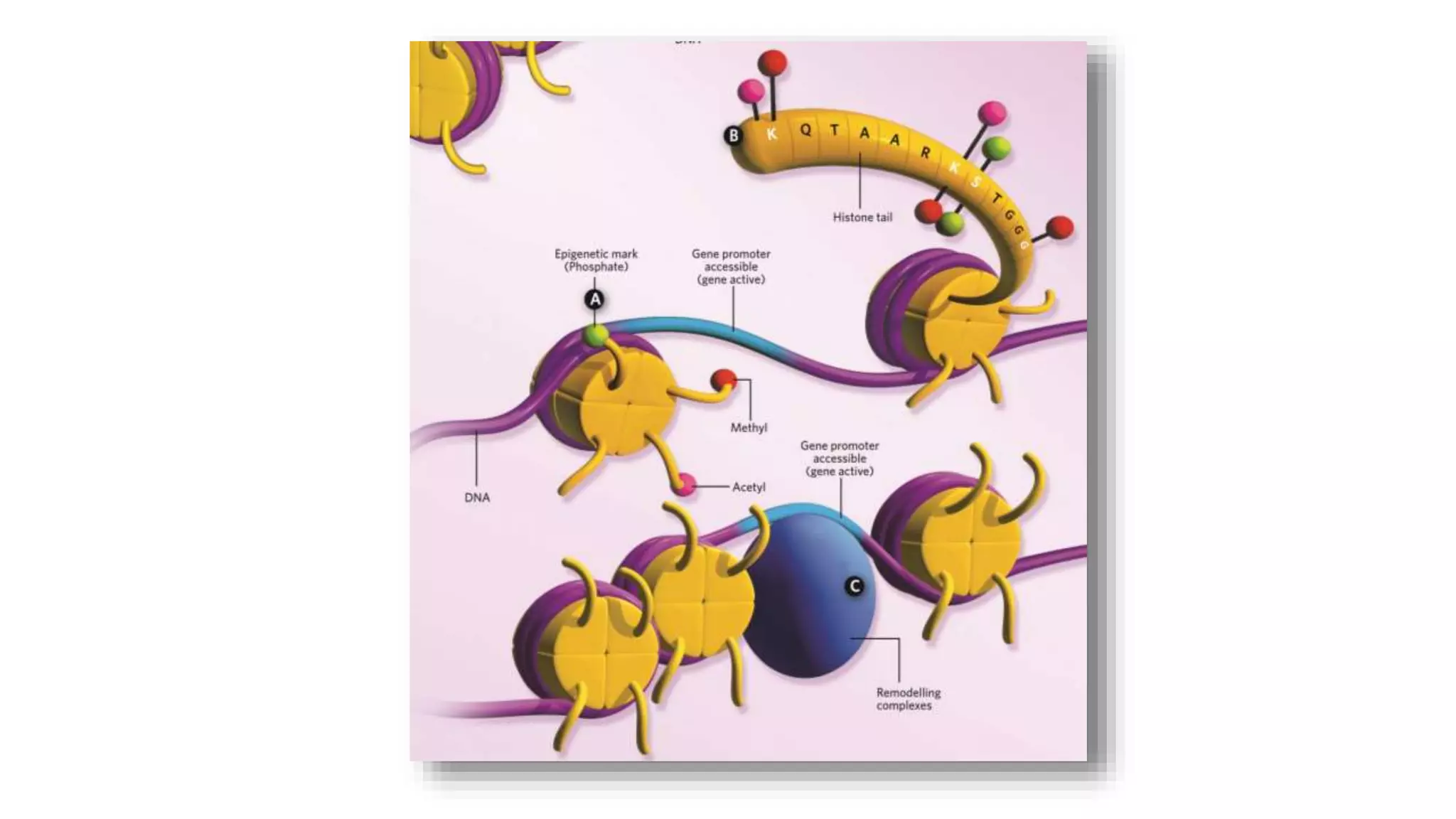
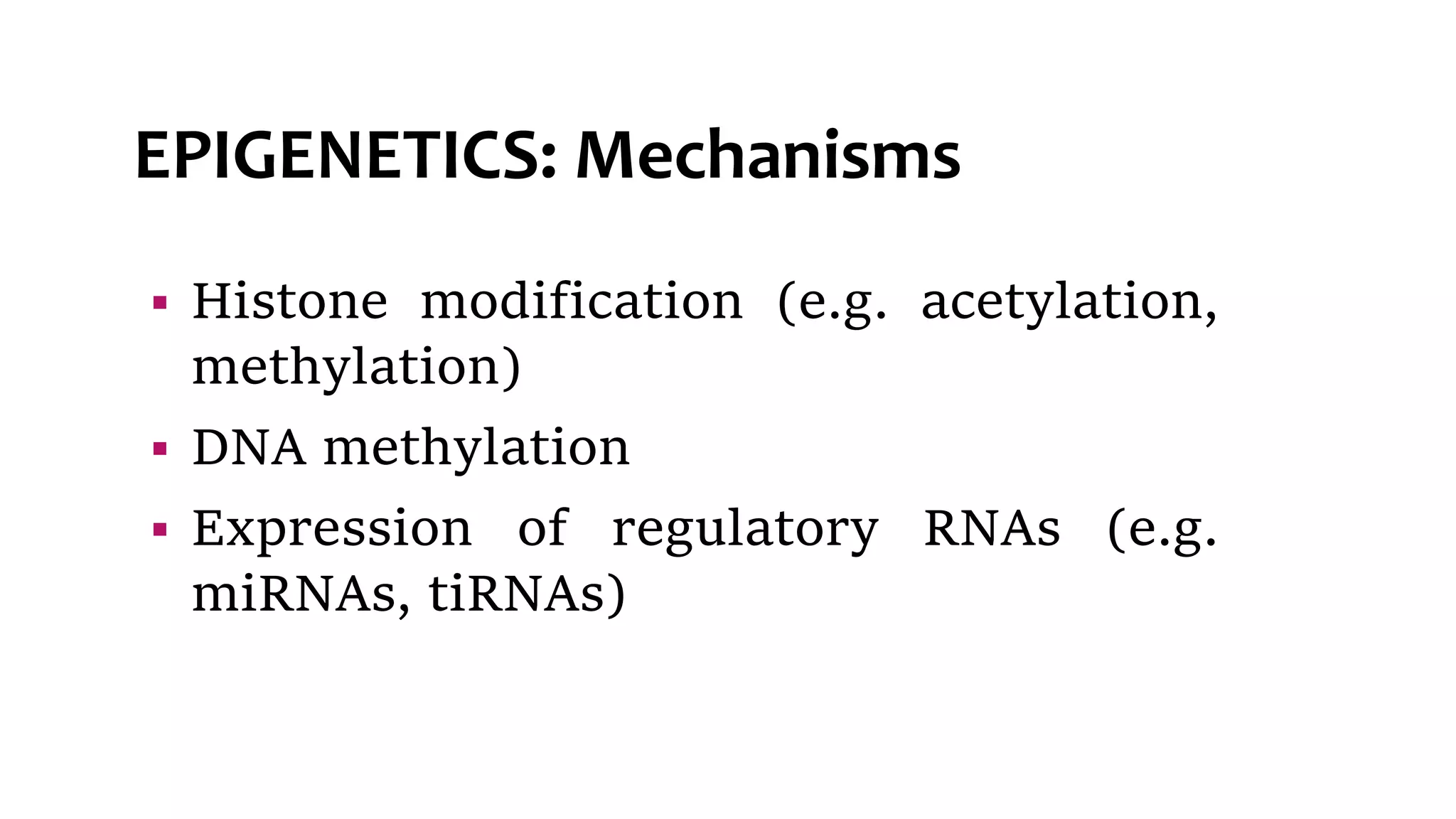
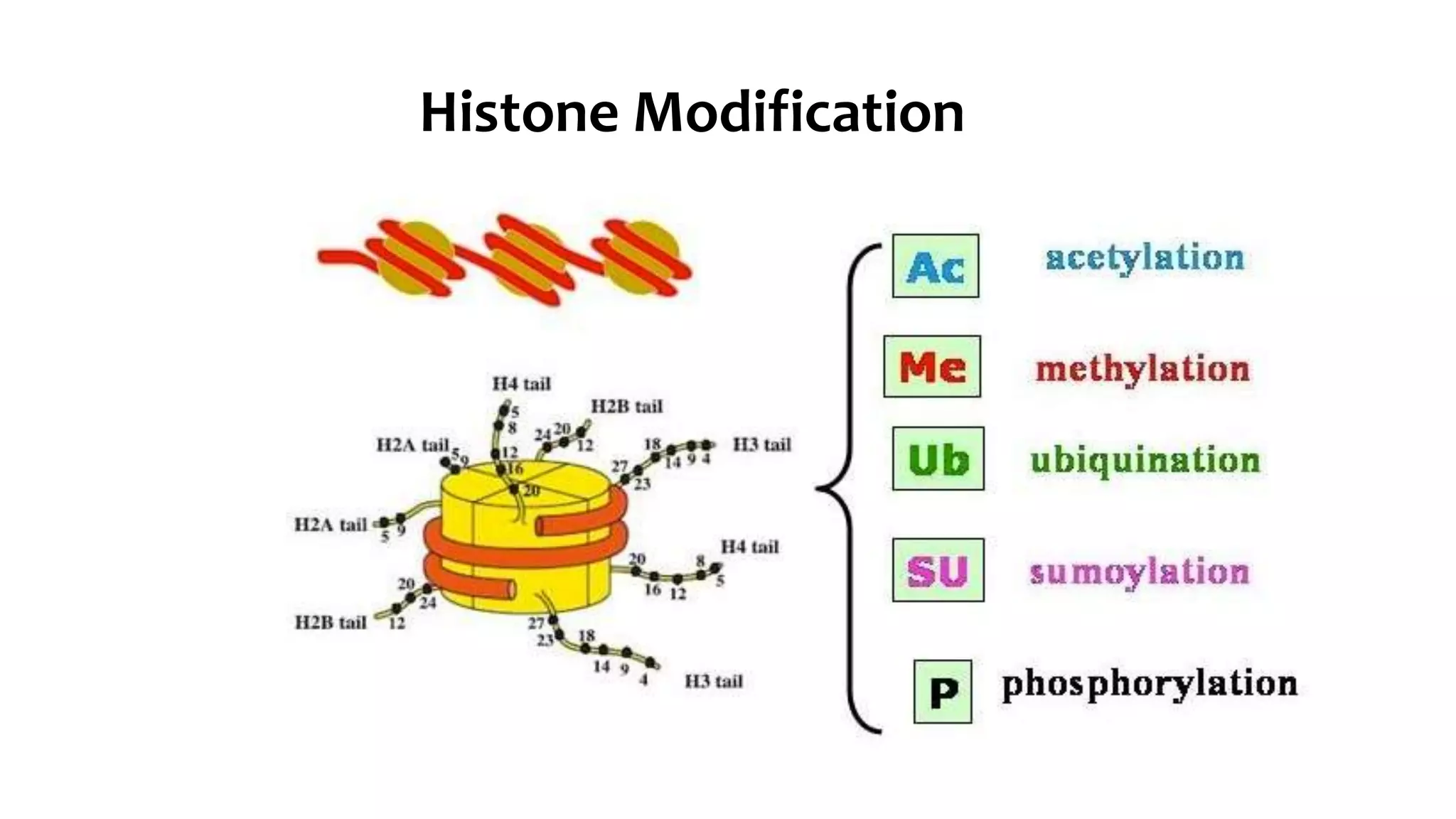
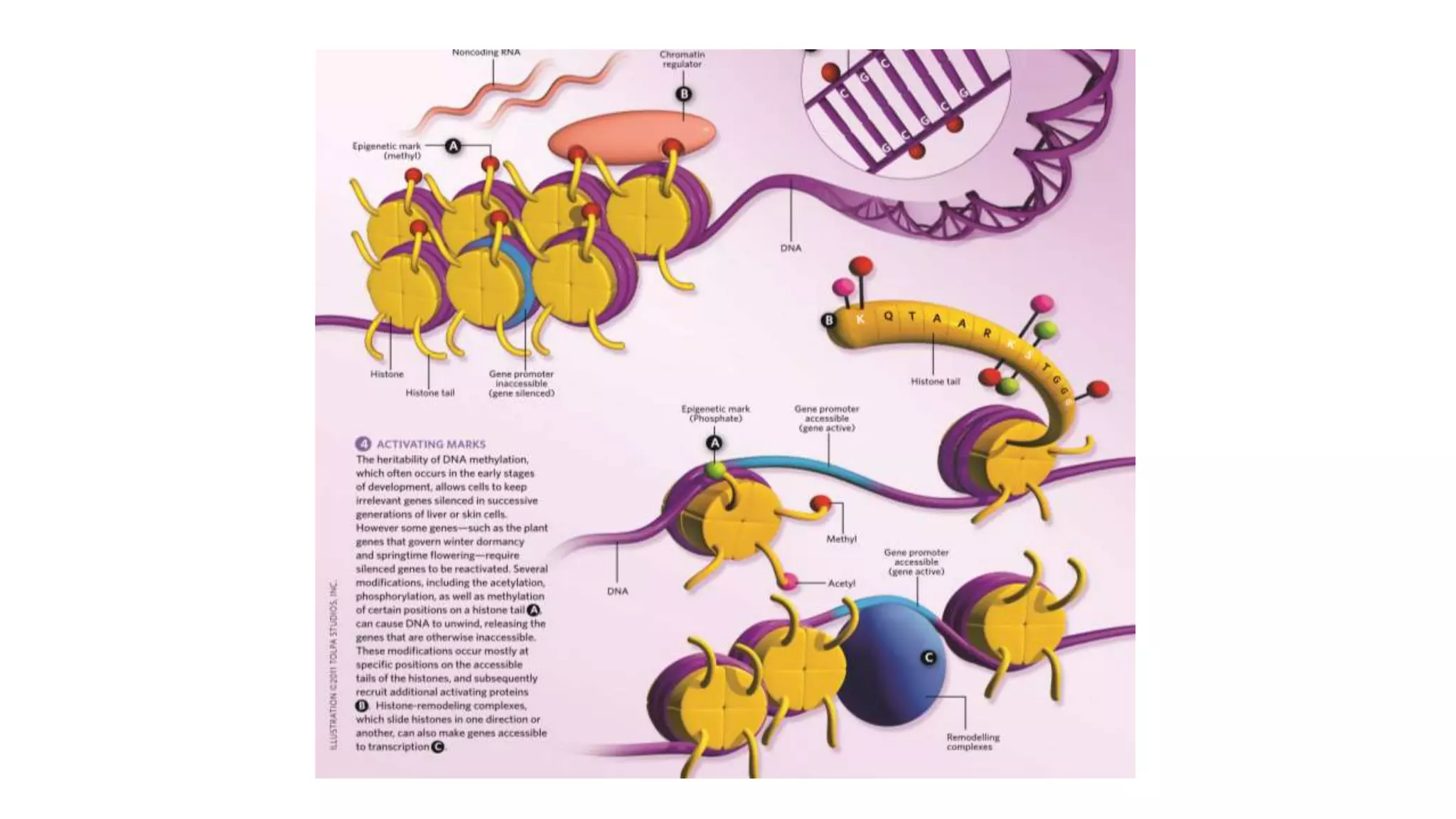
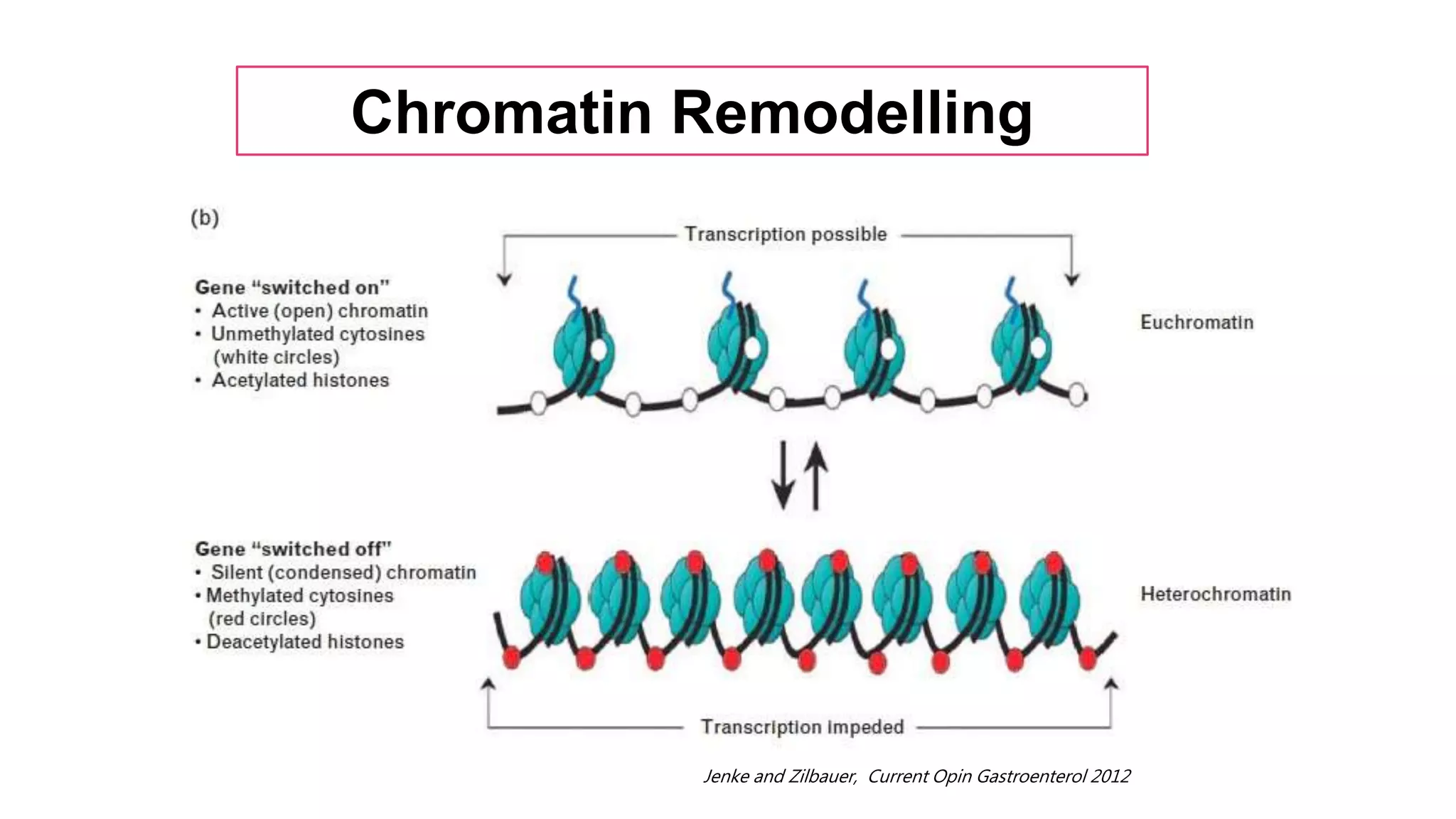
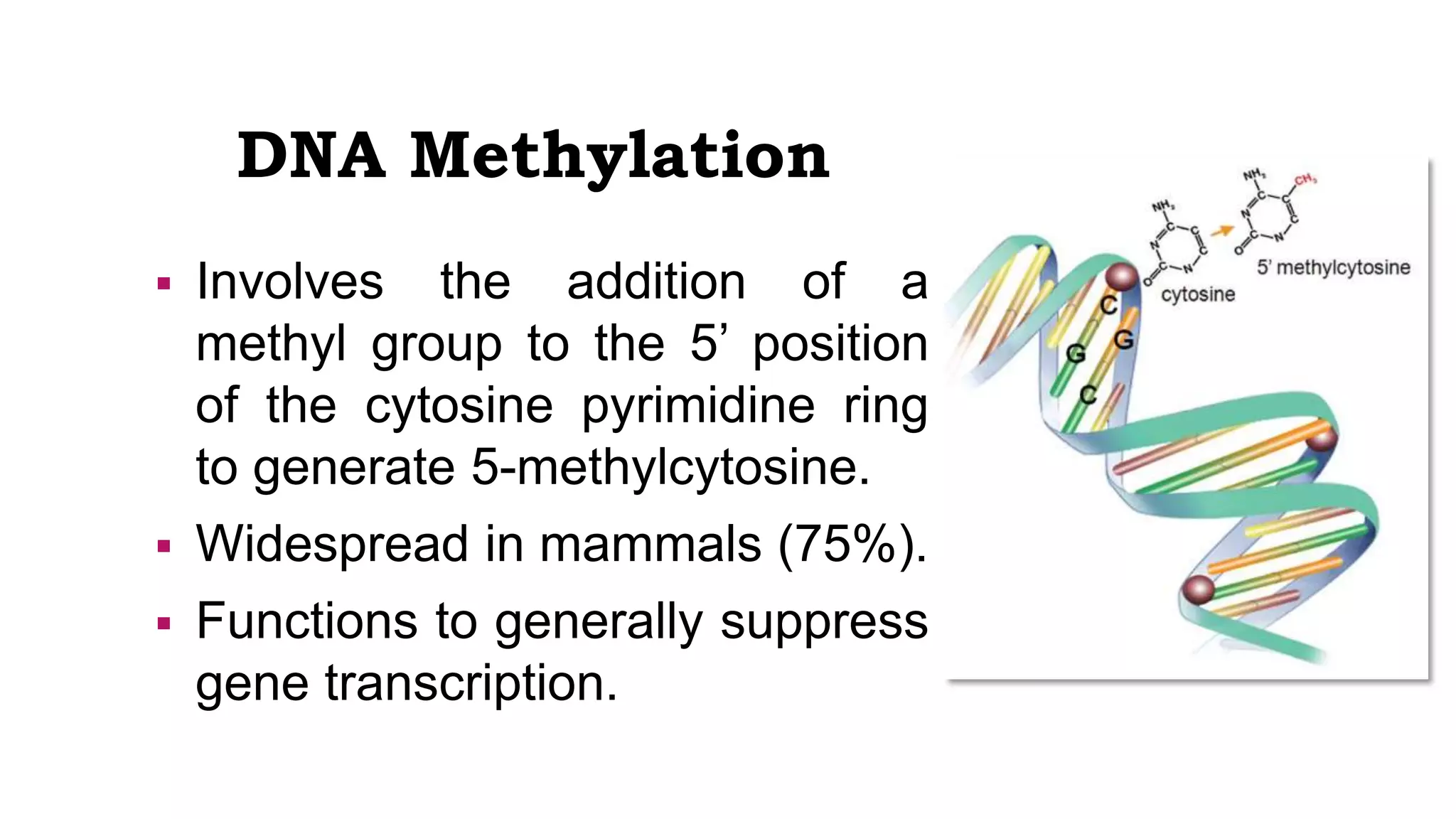
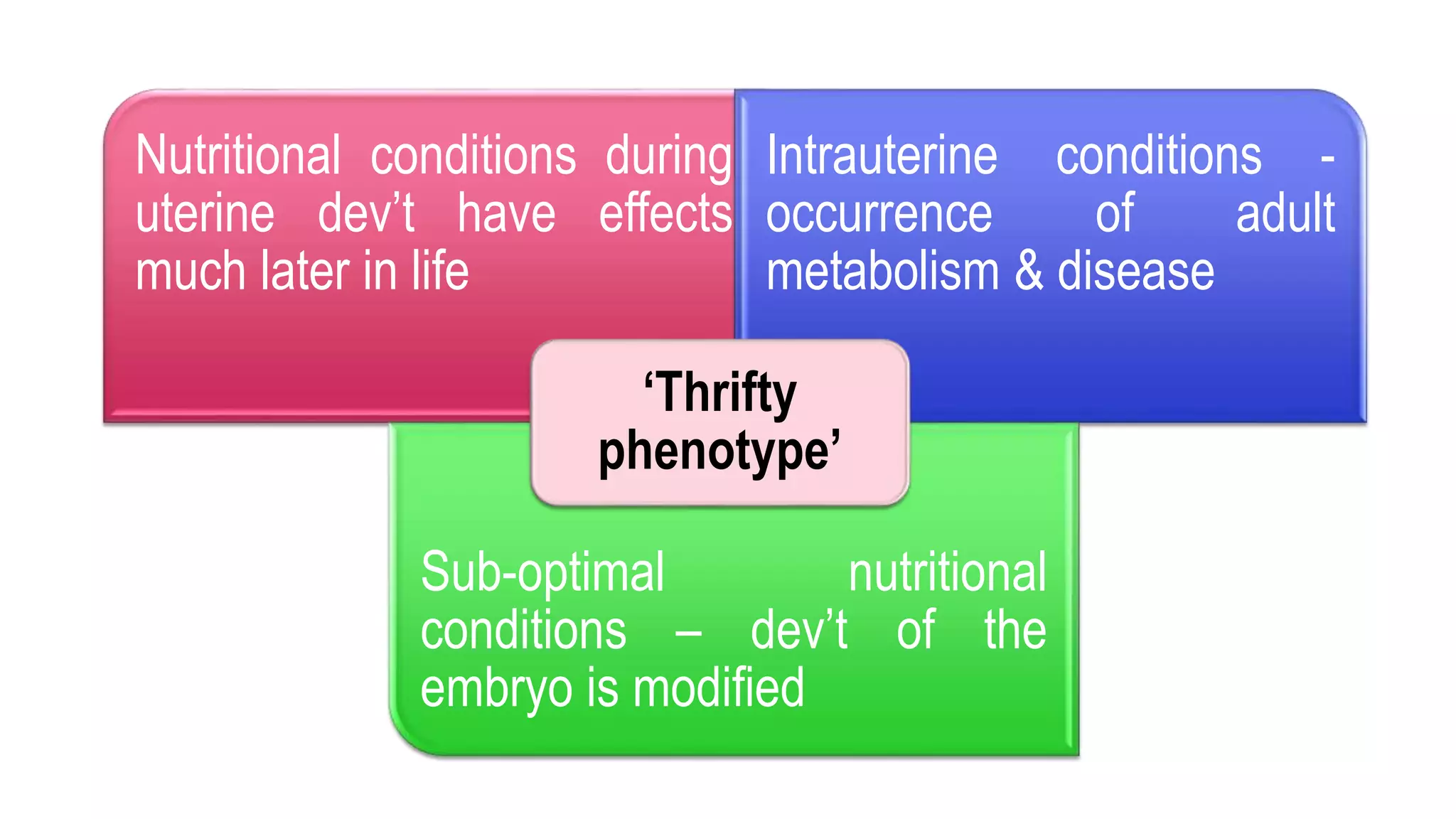
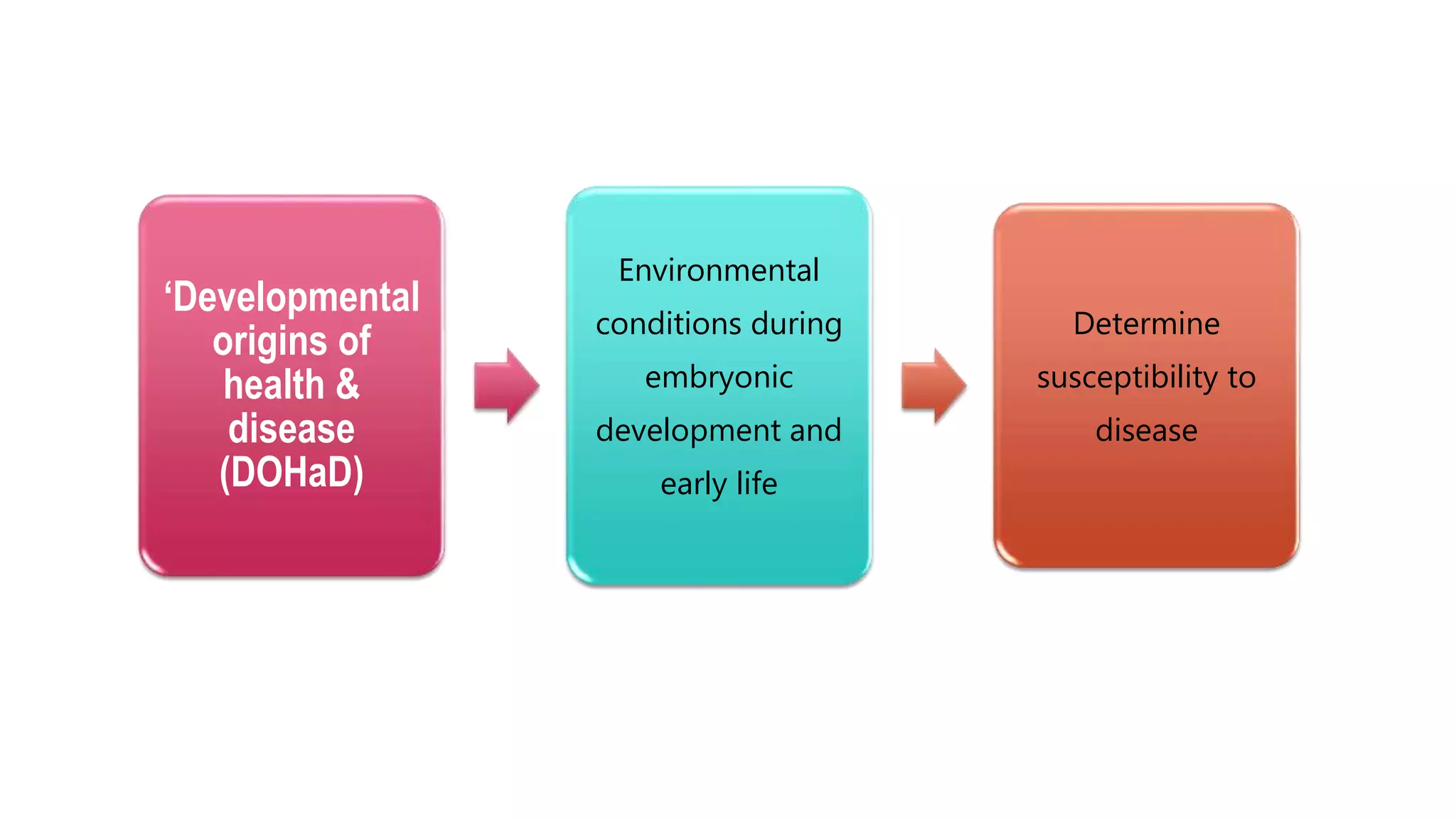
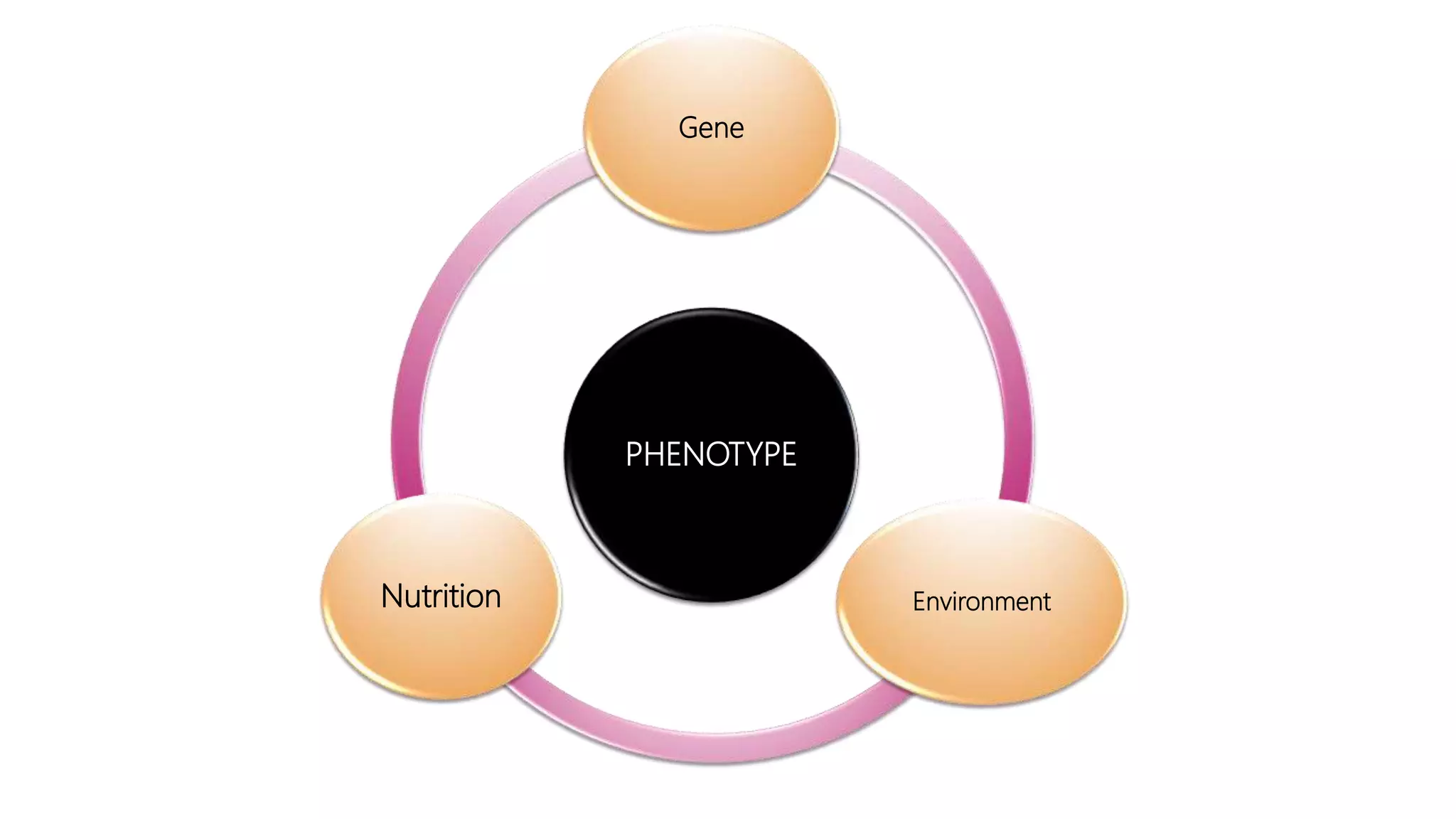
This document provides an overview of epigenetics, detailing its role in heritable changes in phenotype without altering DNA sequences. It discusses various mechanisms such as DNA methylation, histone modifications, and the influence of non-coding RNAs on gene expression, along with how environmental factors and nutrition impact epigenetic alterations. The document also highlights the implications of epigenetics in developmental biology, animal welfare, and fertility in dairy animals.