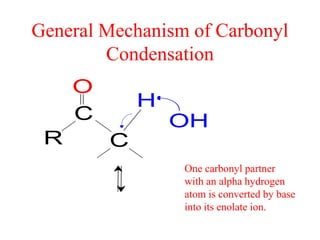
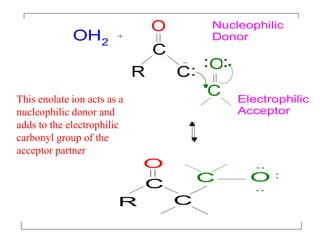
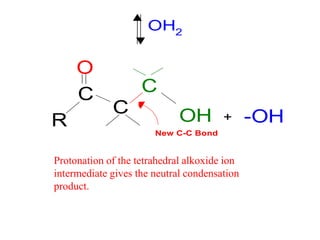
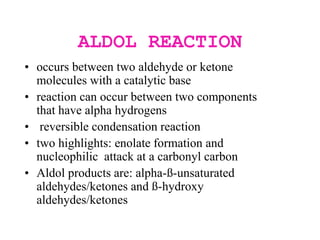
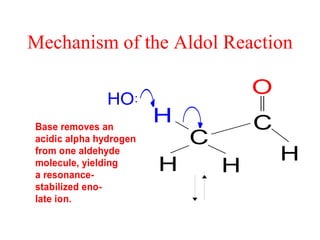
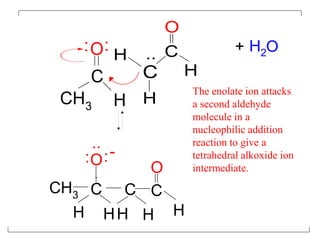
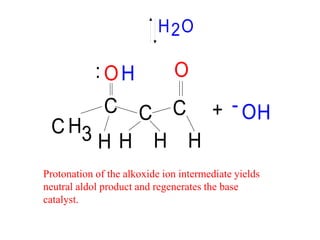
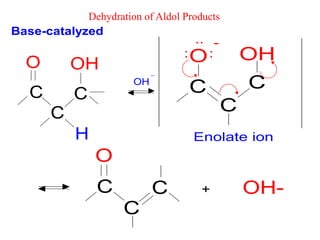
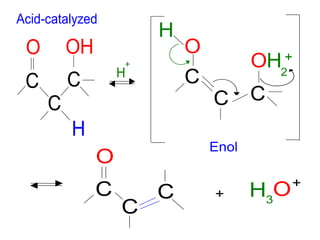
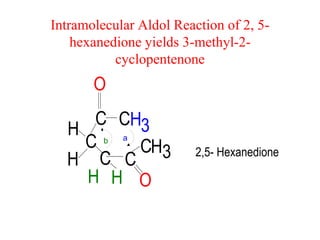
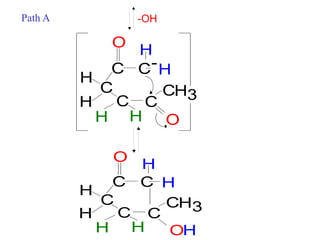
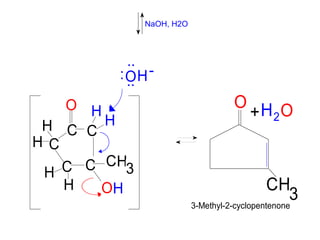
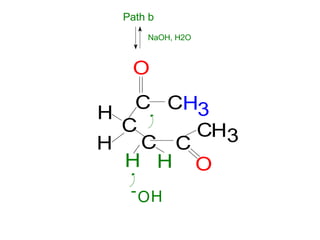
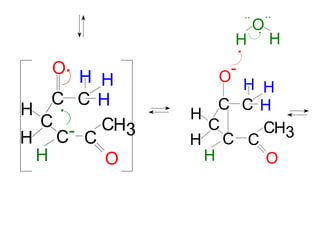
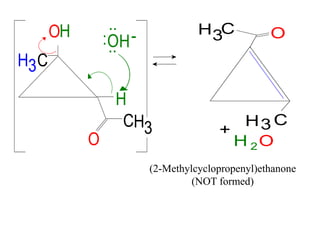
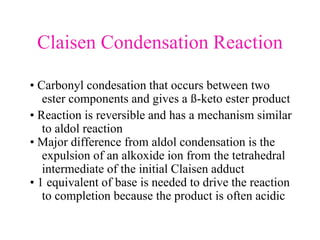
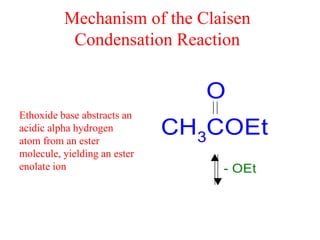
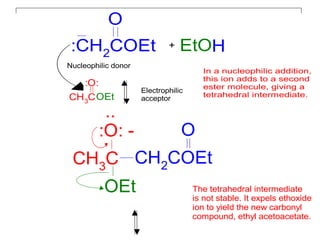
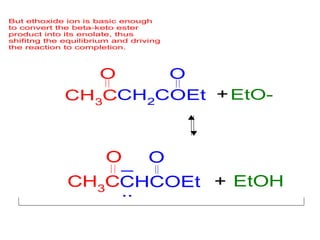
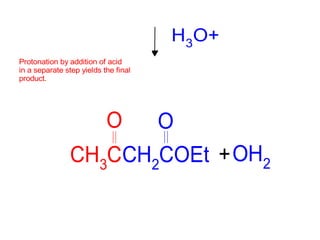
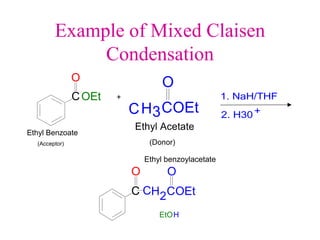
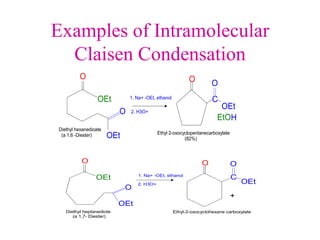
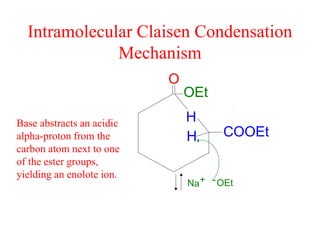
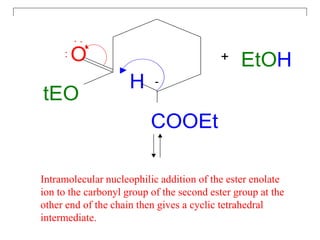
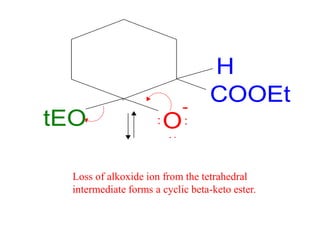
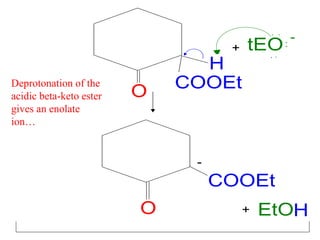
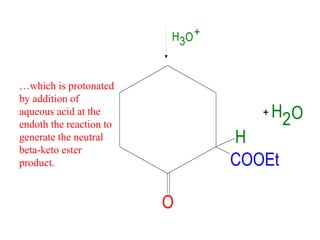
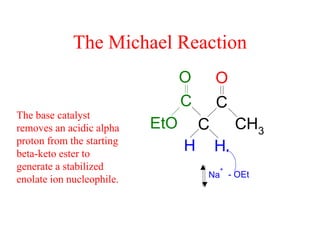
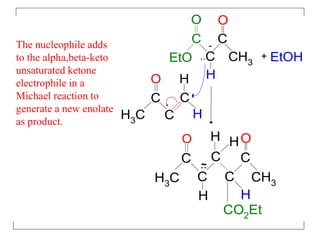
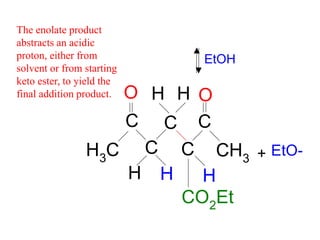
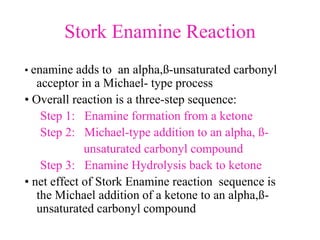
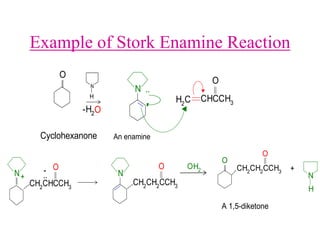
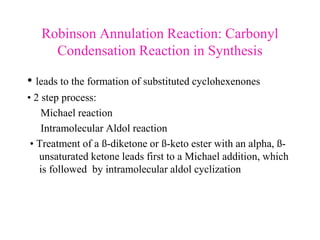
The document discusses various carbonyl condensation reactions including aldol reactions, Claisen condensations, Michael additions, and the Robinson annulation reaction. These reactions involve the nucleophilic addition of carbonyl compounds to alpha,beta-unsaturated carbonyl systems, forming new carbon-carbon bonds. Key steps include enolate formation, Michael addition, aldol condensation, and dehydration. Products include alkenes, cyclic ketones, and beta-keto systems. Intramolecular variants allow for carbocyclic ring formation.