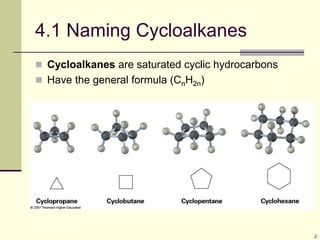
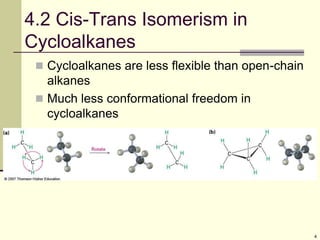
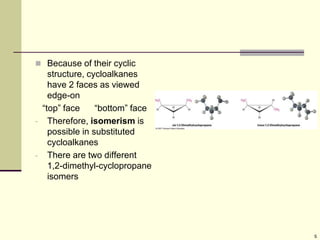
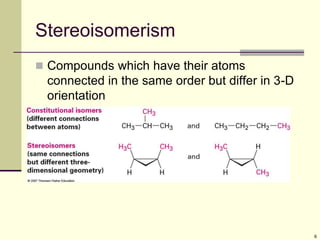
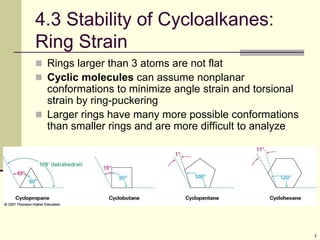
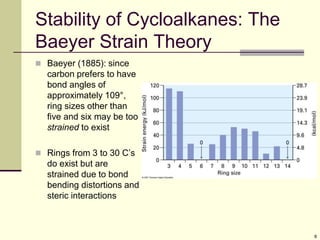
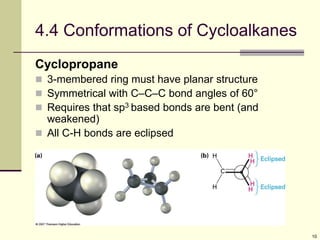
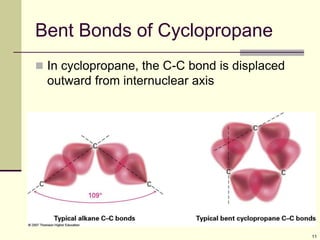
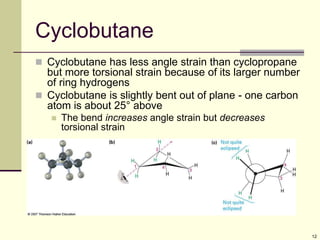
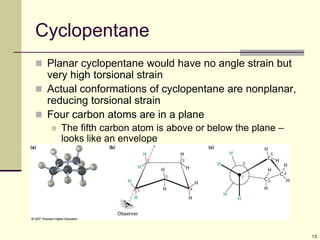
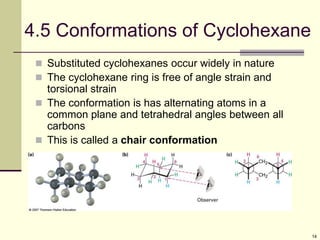
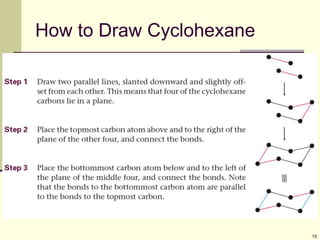
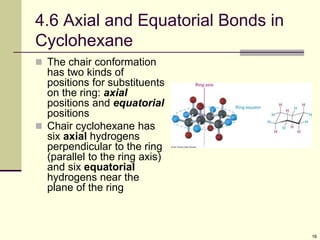
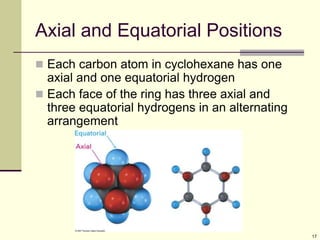
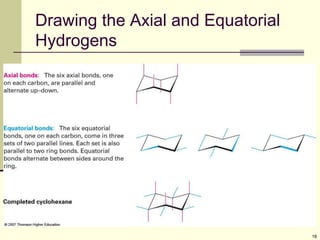
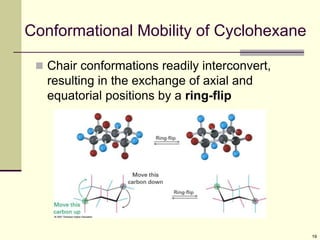
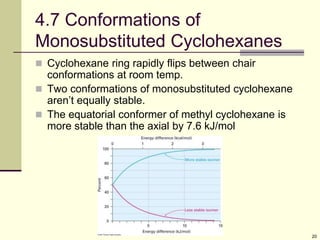
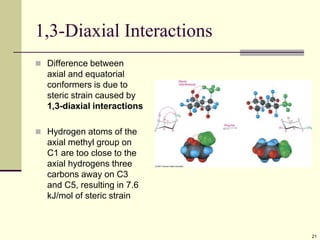
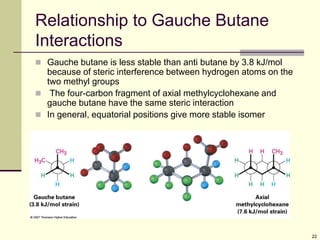
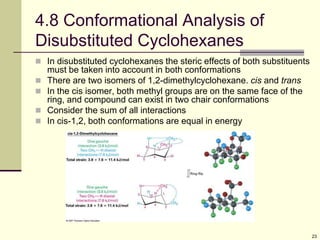
Cycloalkanes are cyclic hydrocarbons that exhibit ring strain due to bond angles deviating from the optimal 109 degrees. Larger rings experience more strain. Cyclohexane minimizes strain by adopting a chair conformation, with axial and equatorial positions for substituents. Monosubstituted cyclohexanes prefer an equatorial position to avoid 1,3-diaxial interactions. In trans-disubstituted cyclohexanes, the diequatorial conformation is most stable by placing substituents in equatorial positions to minimize steric strain.