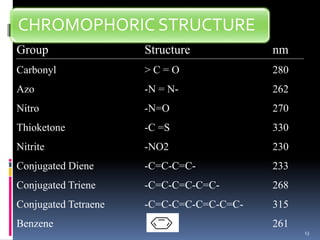
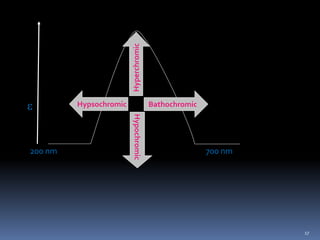
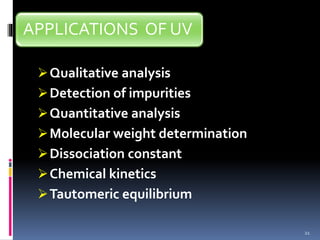
This document discusses absorption laws, chromophores, and limitations in ultraviolet-visible spectroscopy. It describes Beer's law and Lambert's law, which state that absorbance is directly proportional to concentration and path length. Deviations from these laws can occur. Chromophores are groups that absorb specific wavelengths, while auxochromes induce bathochromic shifts. Substituents can cause bathochromic, hypsochromic, hyperchromic, or hypochromic shifts in absorption. UV-Vis spectroscopy has many applications in qualitative and quantitative analysis.