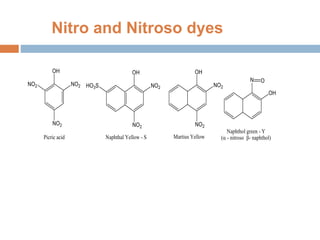
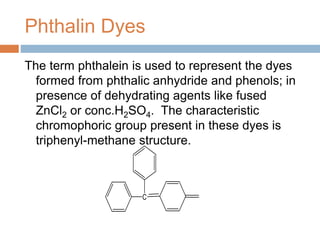
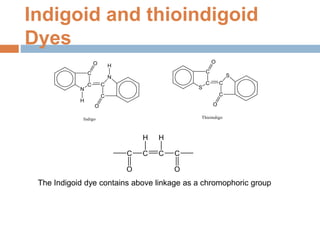
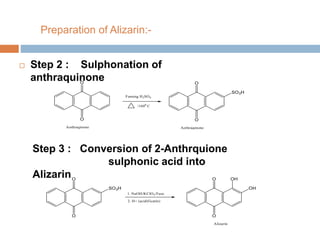
The document provides an overview of dyes, defining them as color substances that can be fixed to various materials like fabrics and paper, emphasizing the importance of chromophores and auxochromes in dye composition. It classifies dyes based on their structure, detailing types such as nitro, azo, triphenylmethane, phthalein, indigoid, thioindigoid, and anthraquinone dyes, along with their properties and usages. Additionally, it includes information on specific dyes like methyl orange, crystal violet, and alizarin, highlighting their preparation methods, applications, and characteristics.