The document discusses key concepts in chemical thermodynamics including:
1) The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another.
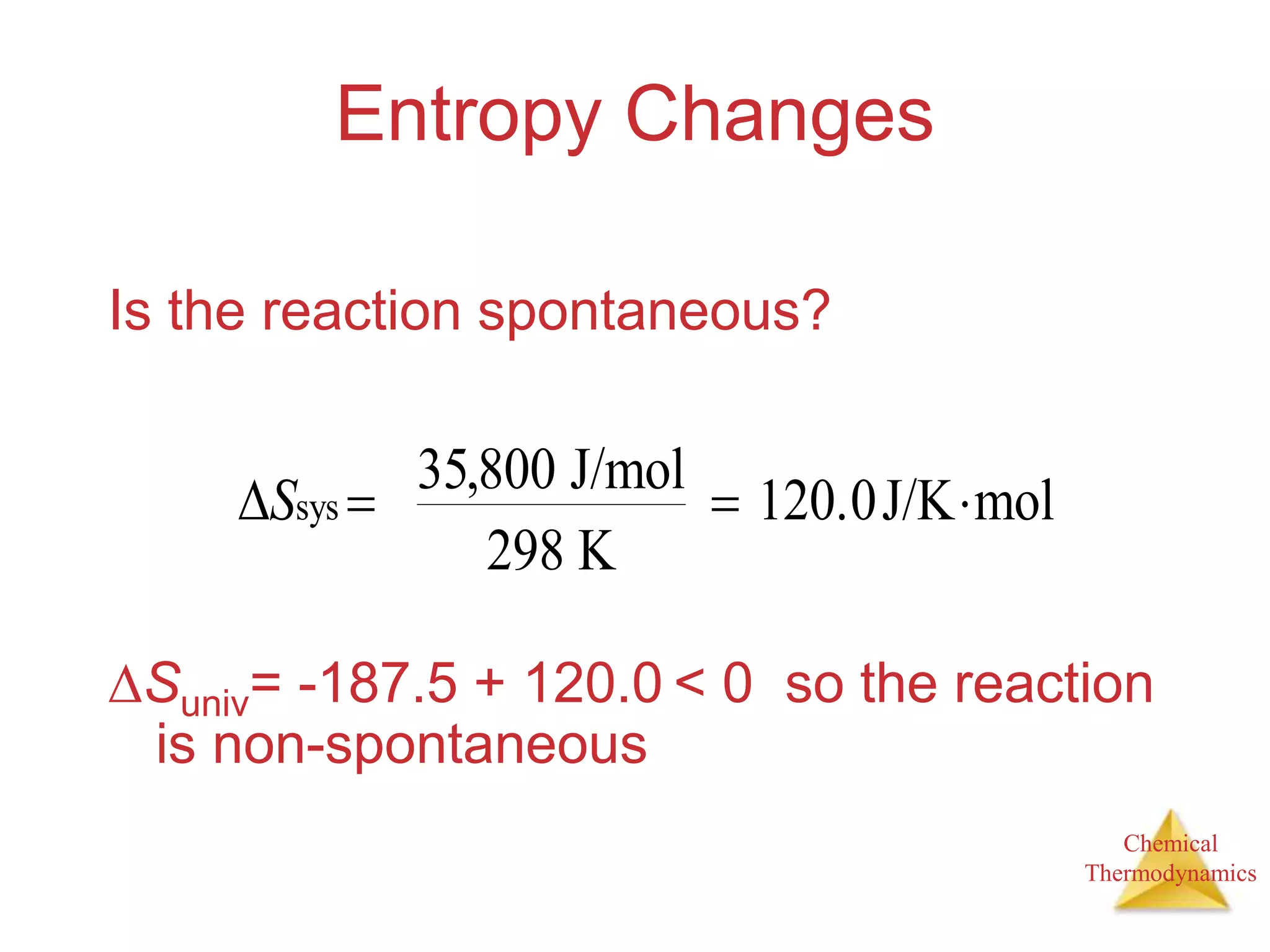
2) Spontaneous processes are those that can proceed without outside intervention. Processes that are spontaneous in one direction are nonspontaneous in the reverse direction.
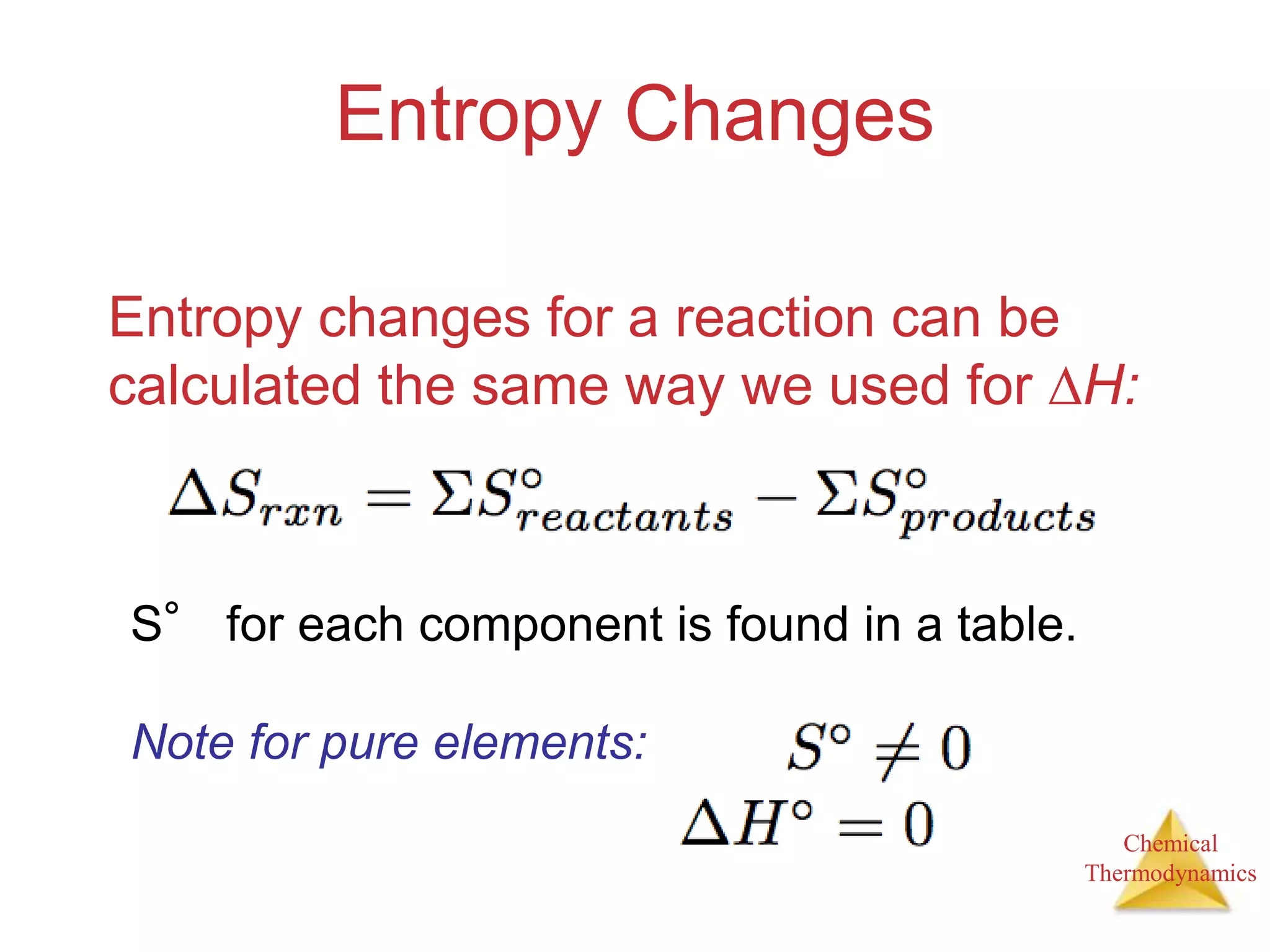
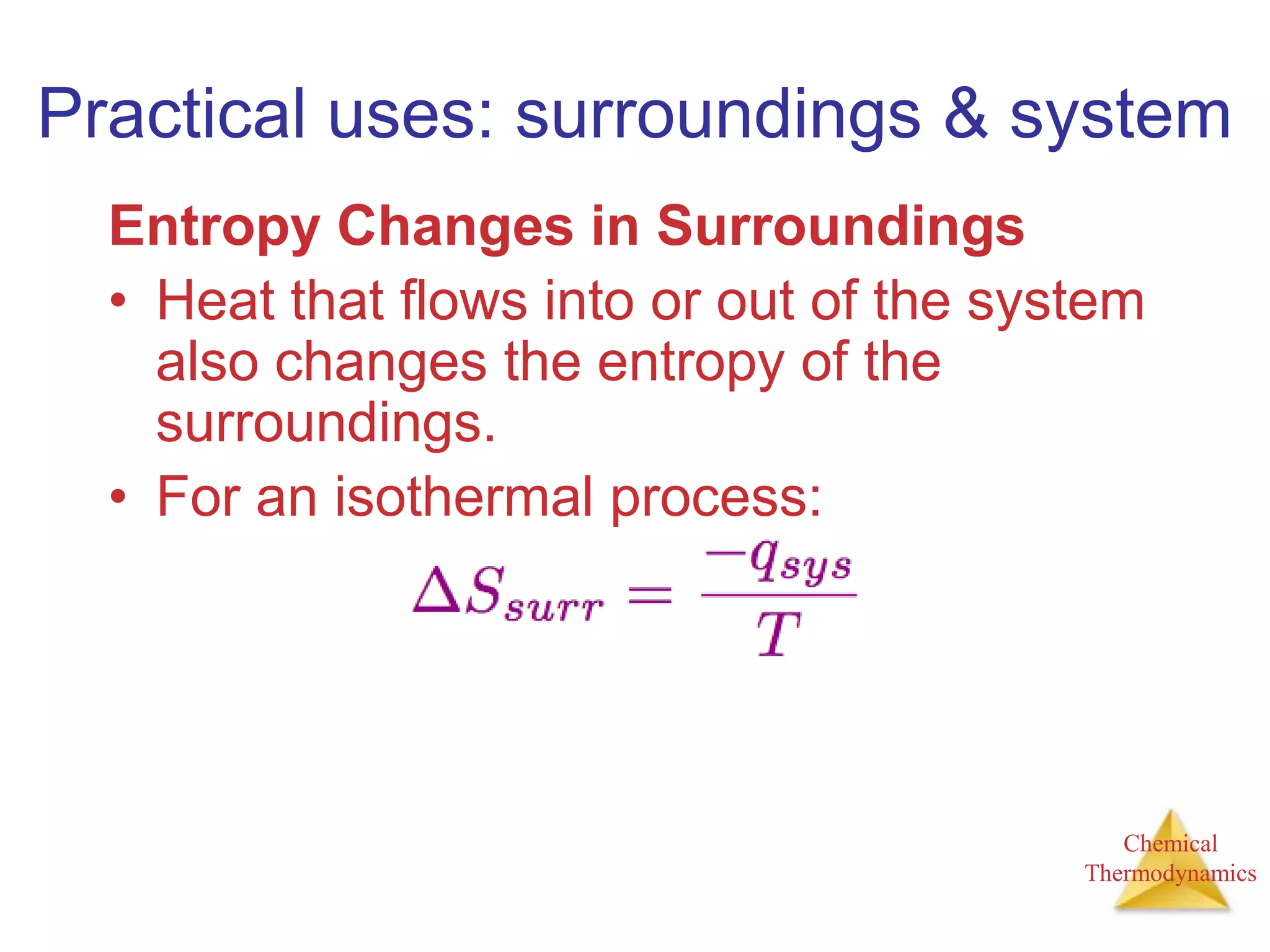
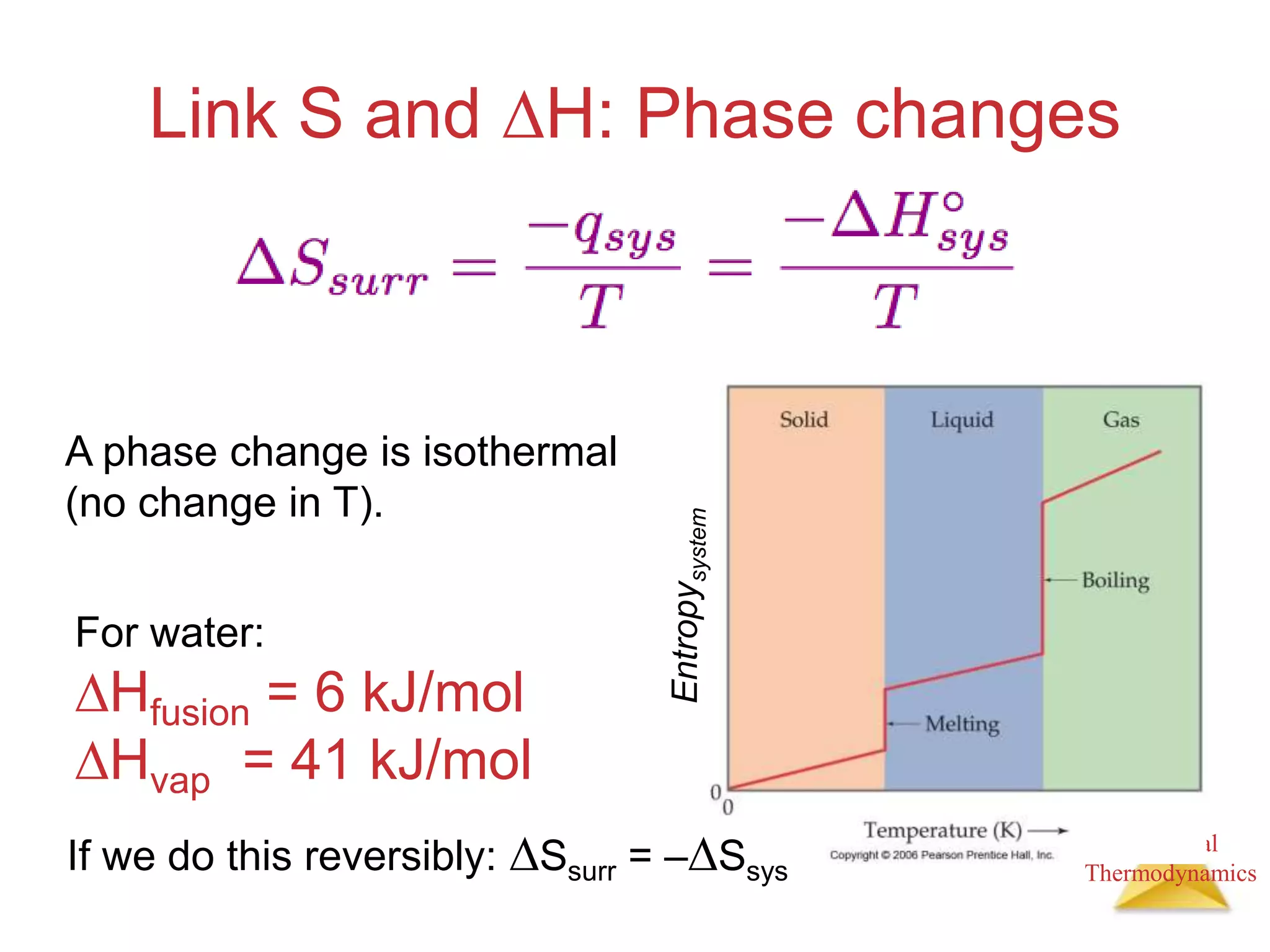
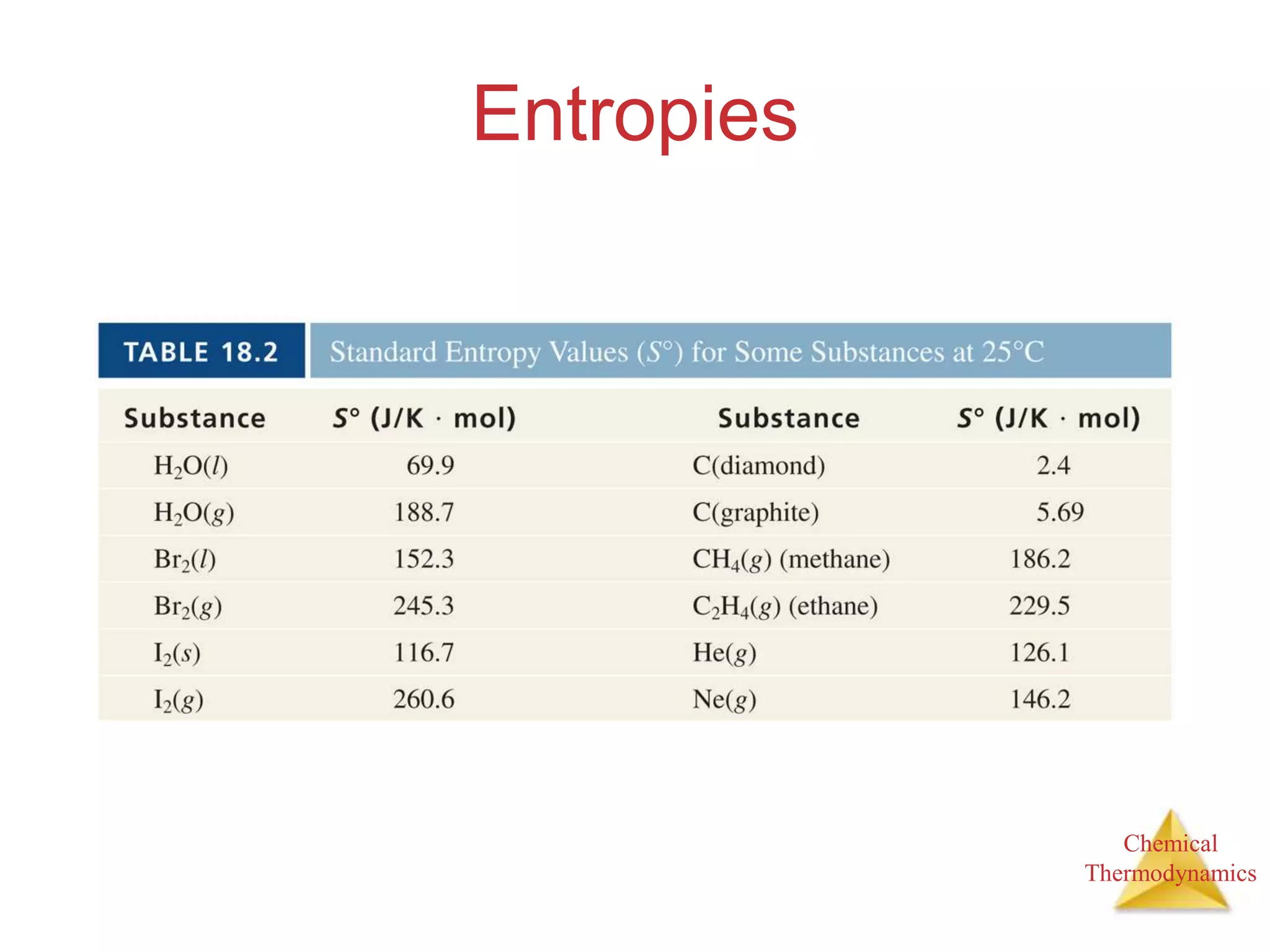
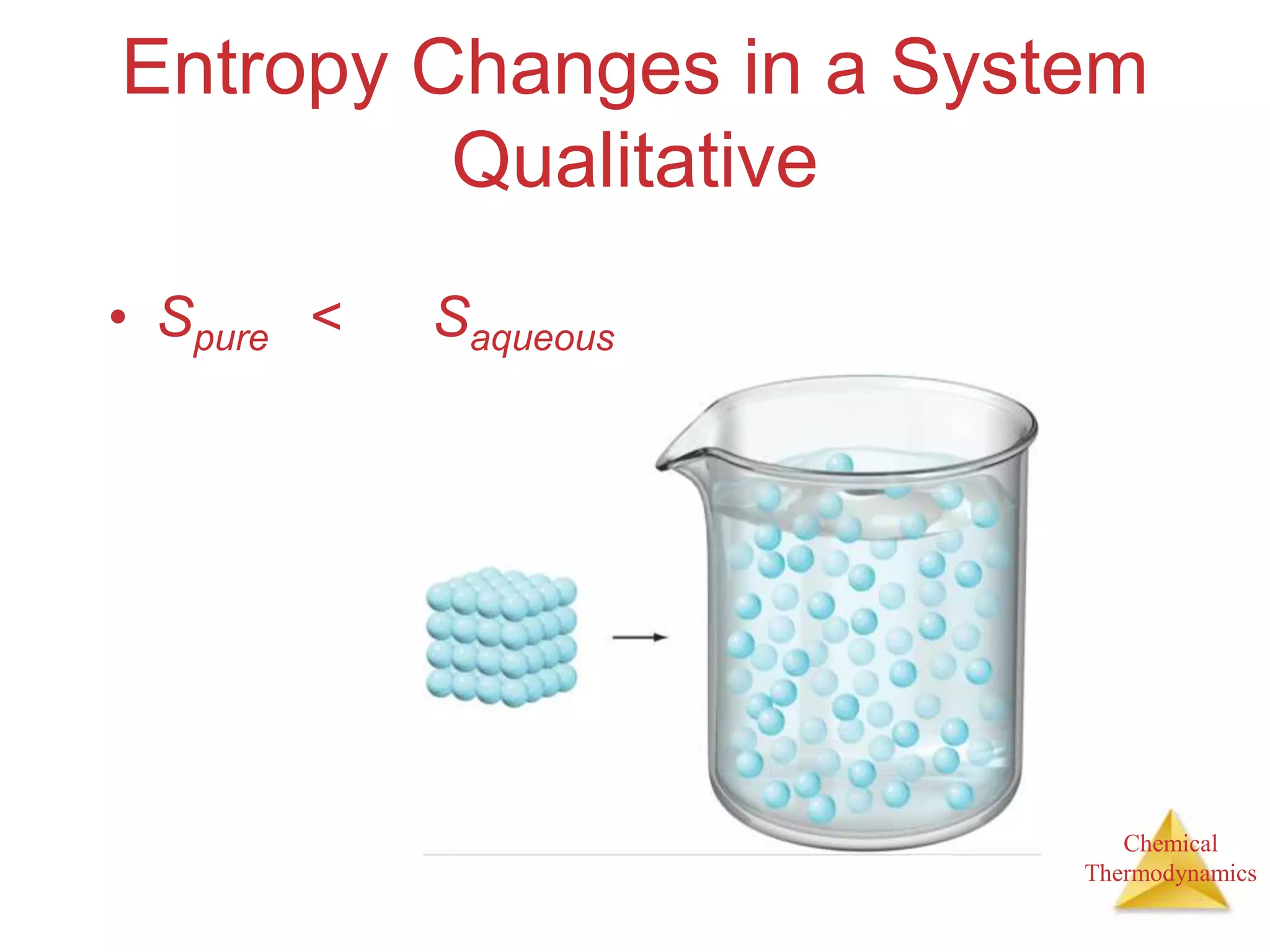
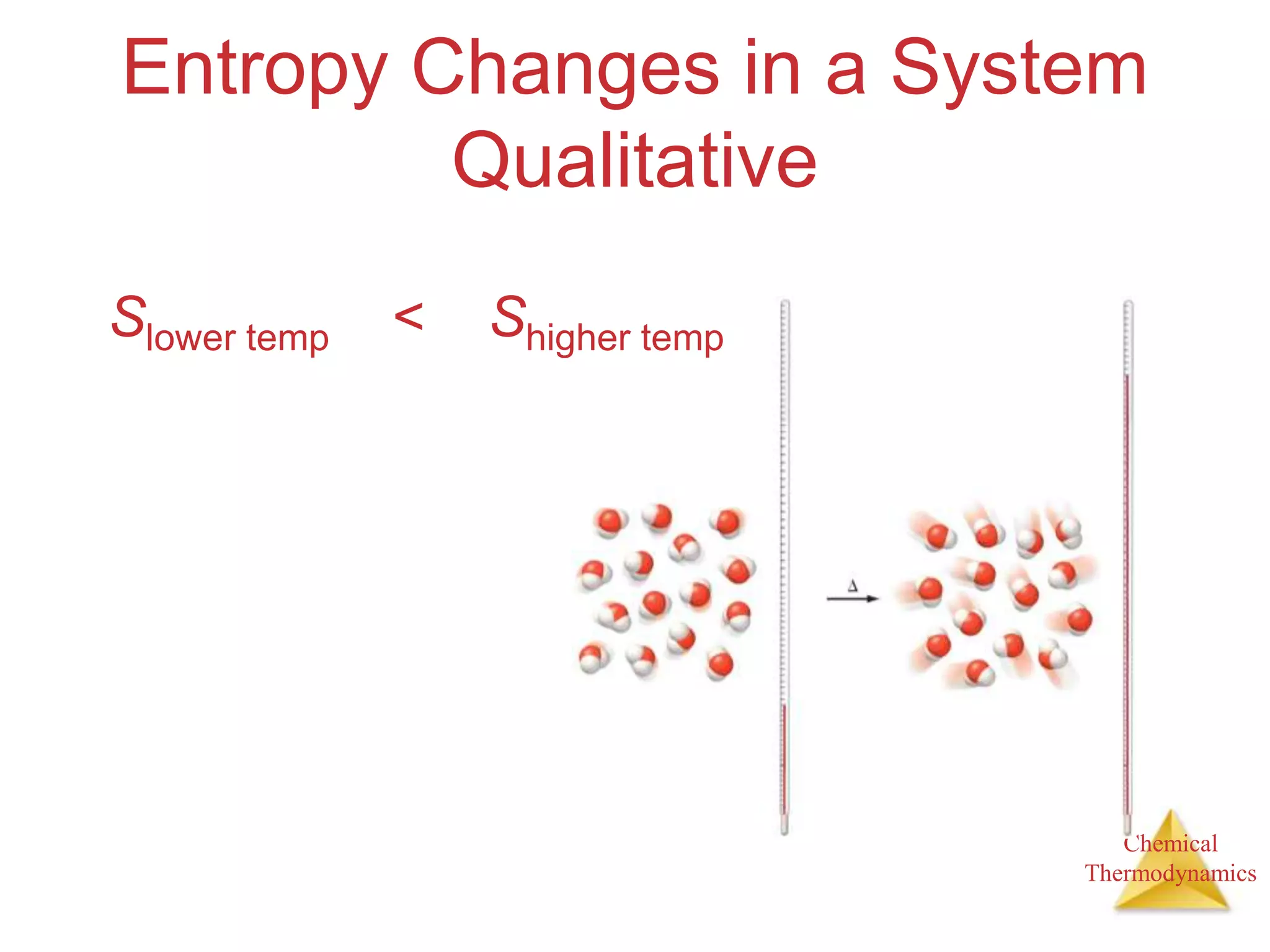
3) Entropy is a measure of the randomness or disorder of a system. It increases for spontaneous processes according to the second law of thermodynamics.
4) The Gibbs free energy, ΔG, can be used to determine whether a process is spontaneous, with a negative ΔG corresponding to a spontaneous process.


































![Chemical
Thermodynamics
Standard Entropy
2NH3(g) N2(g) + 3H2(g)
S
rxn = nS
products mS
reactants
= [(1)(191.5 J/K · mol) + (3)(131.0 J/K · mol)]
- [(2)(193.0 J/K · mol)]
= 584.5 J/K · mol - 386 J/K · mol
S
rxn = 198.5 J/K · mol (Entropy increases)
(2 mol gas 4 mol gas)](https://image.slidesharecdn.com/spontaneity-entropy-and-free-energy-220904080652-d54d7958/75/Spontaneity-Entropy-and-Free-Energy-ppt-42-2048.jpg)


















![Chemical
Thermodynamics
Standard Free Energy Changes
Calculate the standard free-energy
change for the following reaction.
2KClO3(s) 2KCl(s) + 3O2(g)
G
rxn = nG
products mG
reactants
= [2(408.3 kJ/mol) + 3(0)] [2(289.9
kJ/mol)]
= 816.6 (579.8) = 236.8 kJ/mol
(spont)](https://image.slidesharecdn.com/spontaneity-entropy-and-free-energy-220904080652-d54d7958/75/Spontaneity-Entropy-and-Free-Energy-ppt-62-2048.jpg)



![Chemical
Thermodynamics
Free Energy and Equilibrium
2 2
( ) (0.30)
HCl 0.80
( ) ( ) (0.25) (0.45)
H Cl
2 2
P
QP P P
First, calculate standard free energy:
H2(g) + Cl2(g) 2 HCl(g)
G° = [2(95.27 kJ/mol)] [0 + 0] =
190.54 kJ/mol
Second, find Q:](https://image.slidesharecdn.com/spontaneity-entropy-and-free-energy-220904080652-d54d7958/75/Spontaneity-Entropy-and-Free-Energy-ppt-66-2048.jpg)





![Chemical
Thermodynamics
Relationship Between G° and K
First, calculate the G°:
= [0 + 0] [2(95.27 kJ/mol)]
= 190.54 kJ/mol (non-spontaneous)
Substitute into equation:
190.54 kJ/mol = (8.314 x 103 kJ/K·mol)(298
K) ln KP
76.90 = ln KP = 3.98 x 1034
K < 1 reactants are favored](https://image.slidesharecdn.com/spontaneity-entropy-and-free-energy-220904080652-d54d7958/75/Spontaneity-Entropy-and-Free-Energy-ppt-72-2048.jpg)






