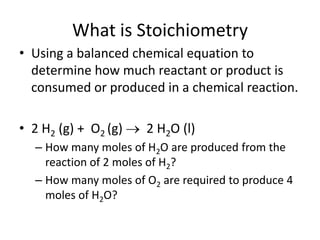
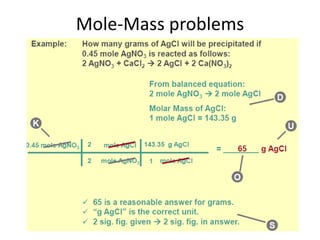
Stoichiometry allows us to use balanced chemical equations to determine the amounts of reactants and products involved in chemical reactions. It treats the chemical equation like a recipe, using mole ratios derived from the coefficients to solve mole-mole, mole-mass, and mass-mass problems. For example, if 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water, how many moles of oxygen are needed to produce 4 moles of water? By using the 1:1 mole ratio of oxygen to water given in the balanced equation, we can determine that 4 moles of oxygen are needed.