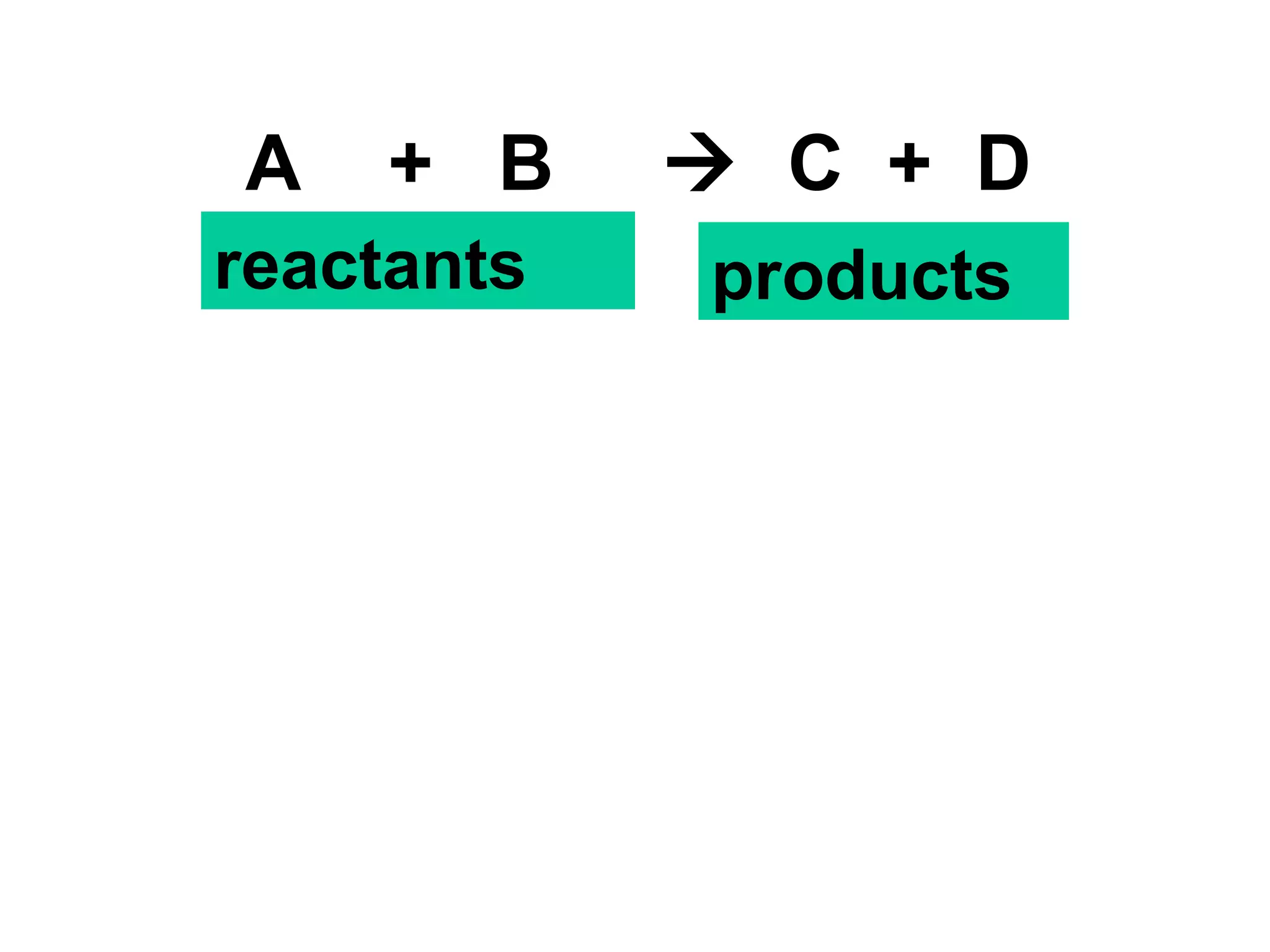
The document discusses key concepts related to chemical reaction rates including:
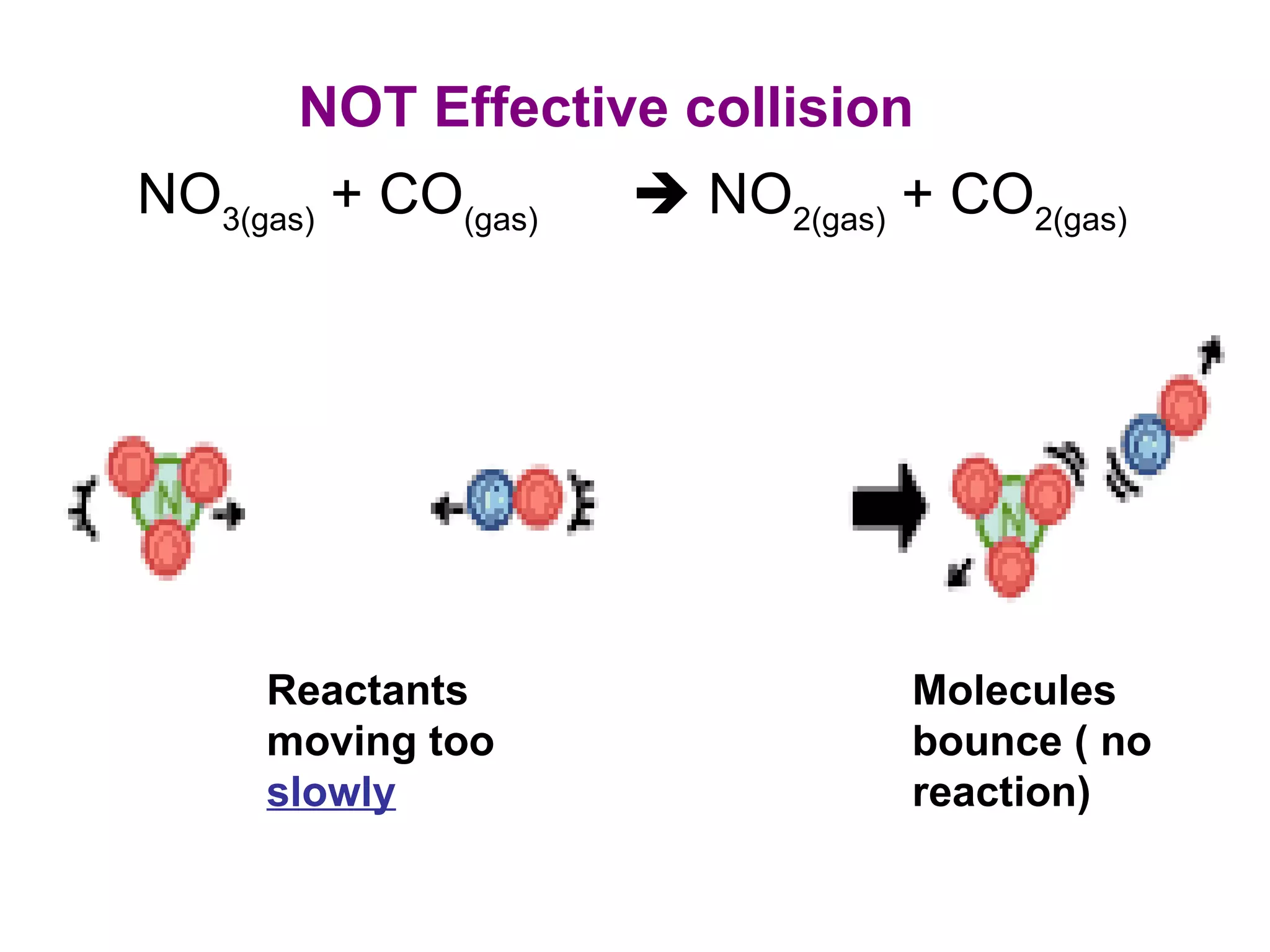
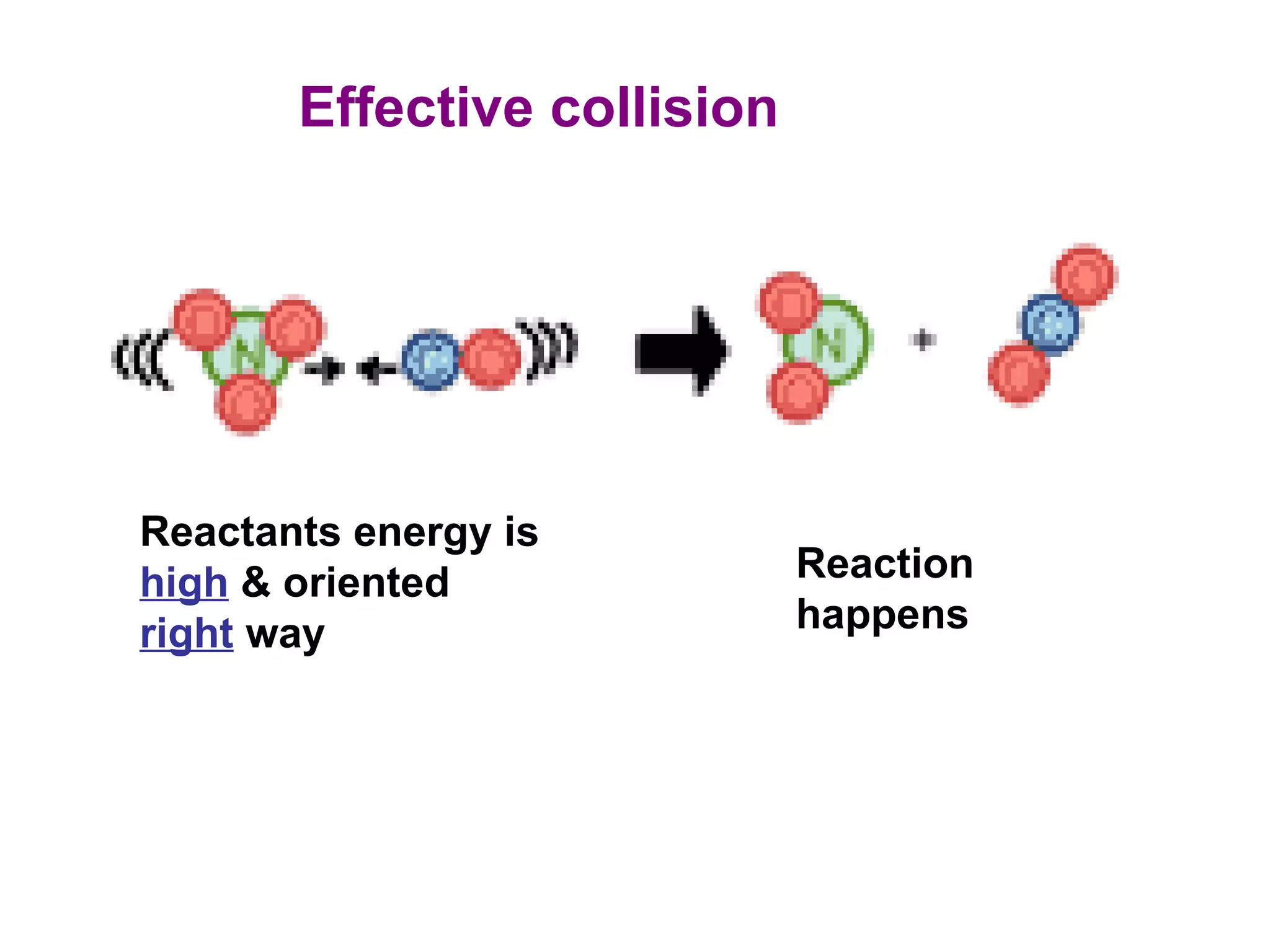
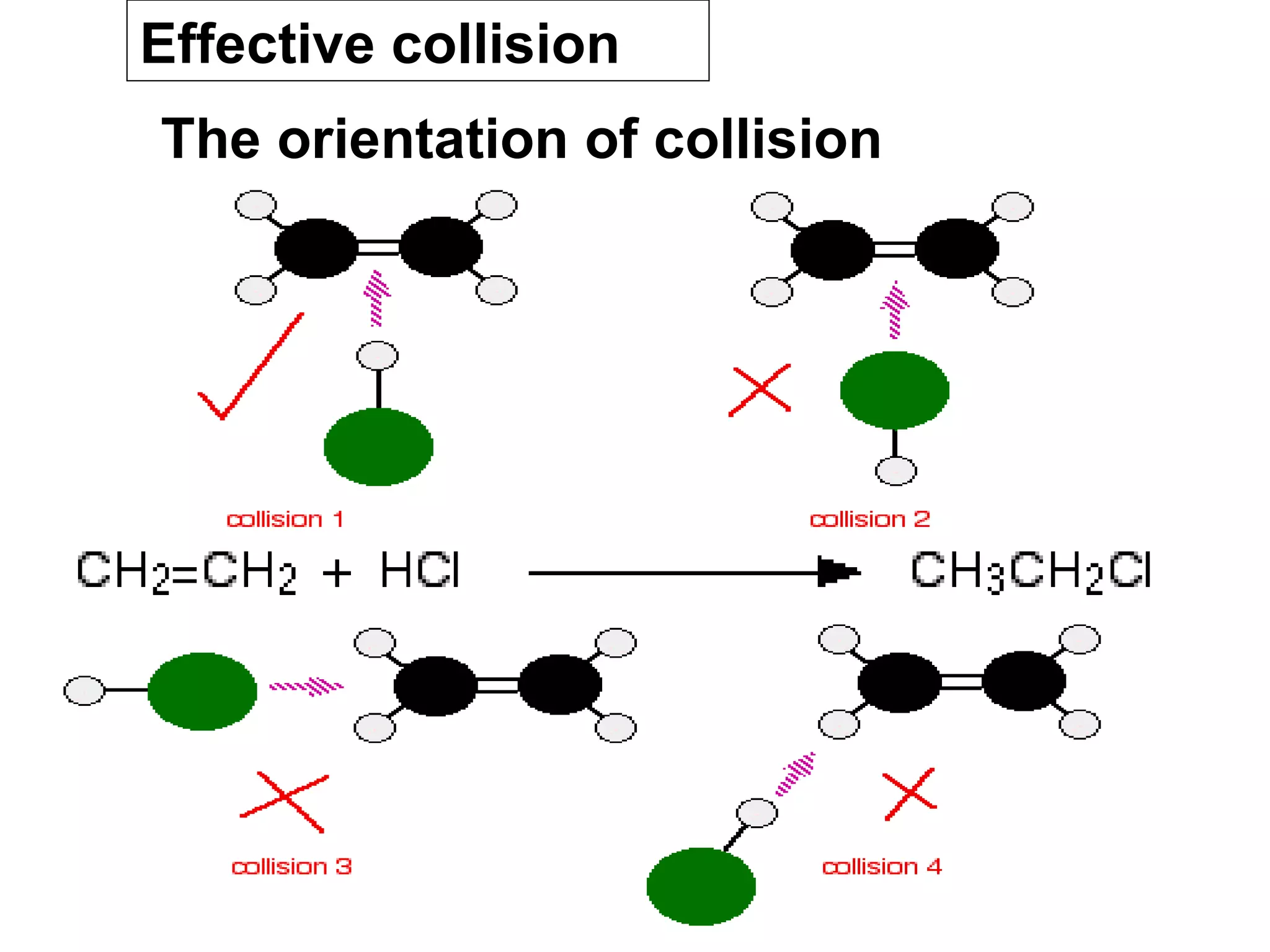
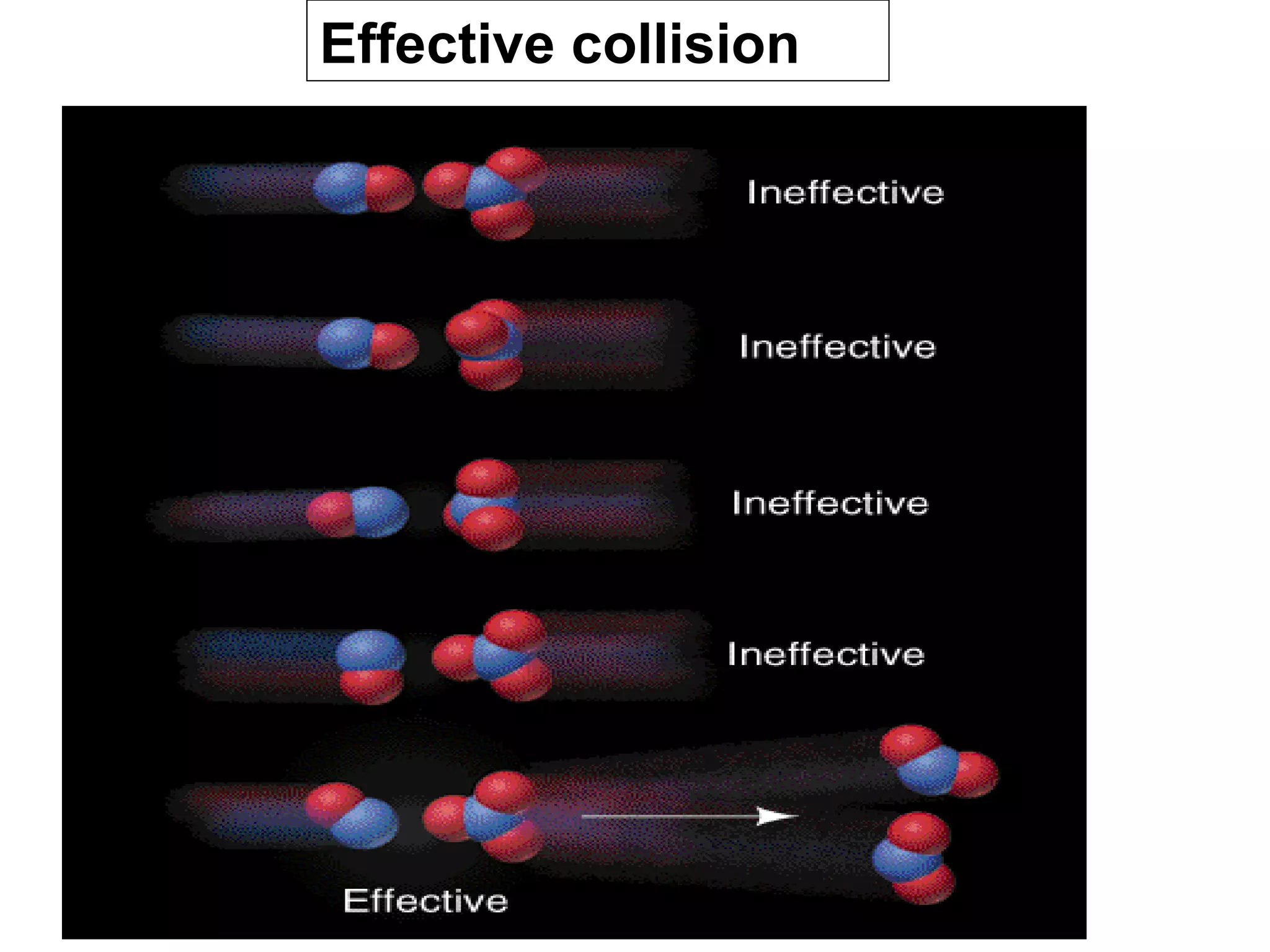
- Collision theory which states that molecules must collide with sufficient energy and correct orientation for a reaction to occur. This is known as an effective collision.
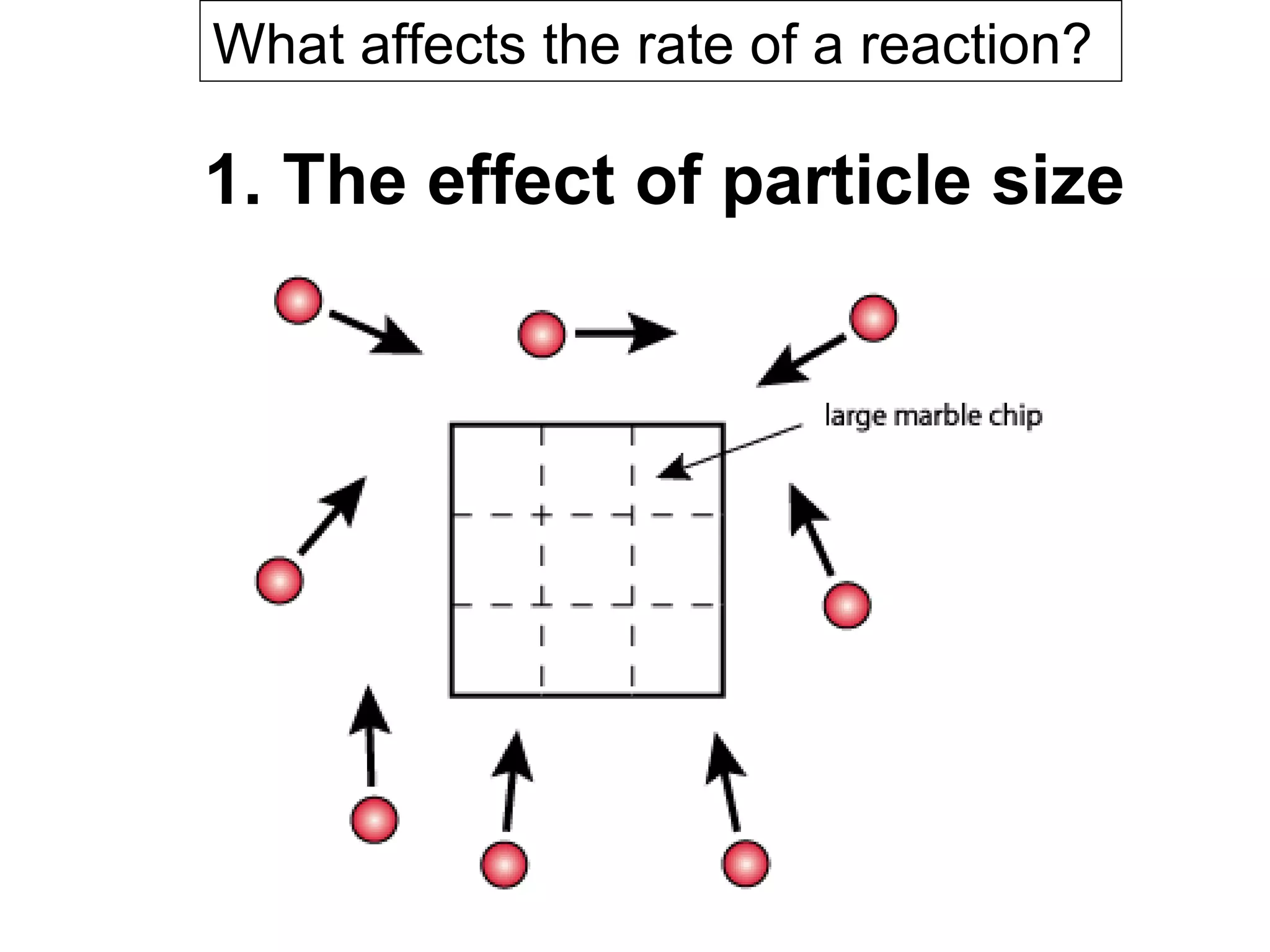
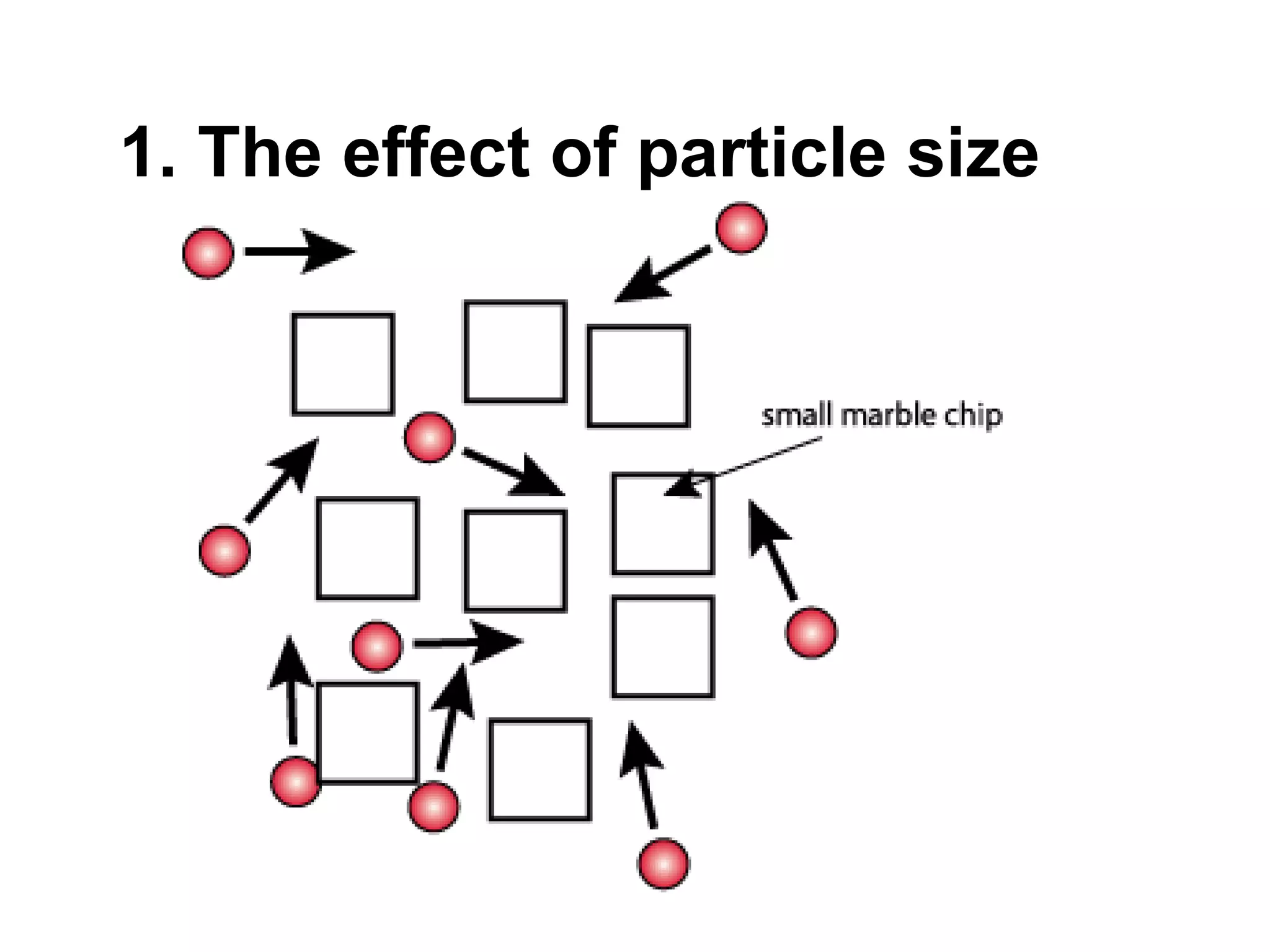
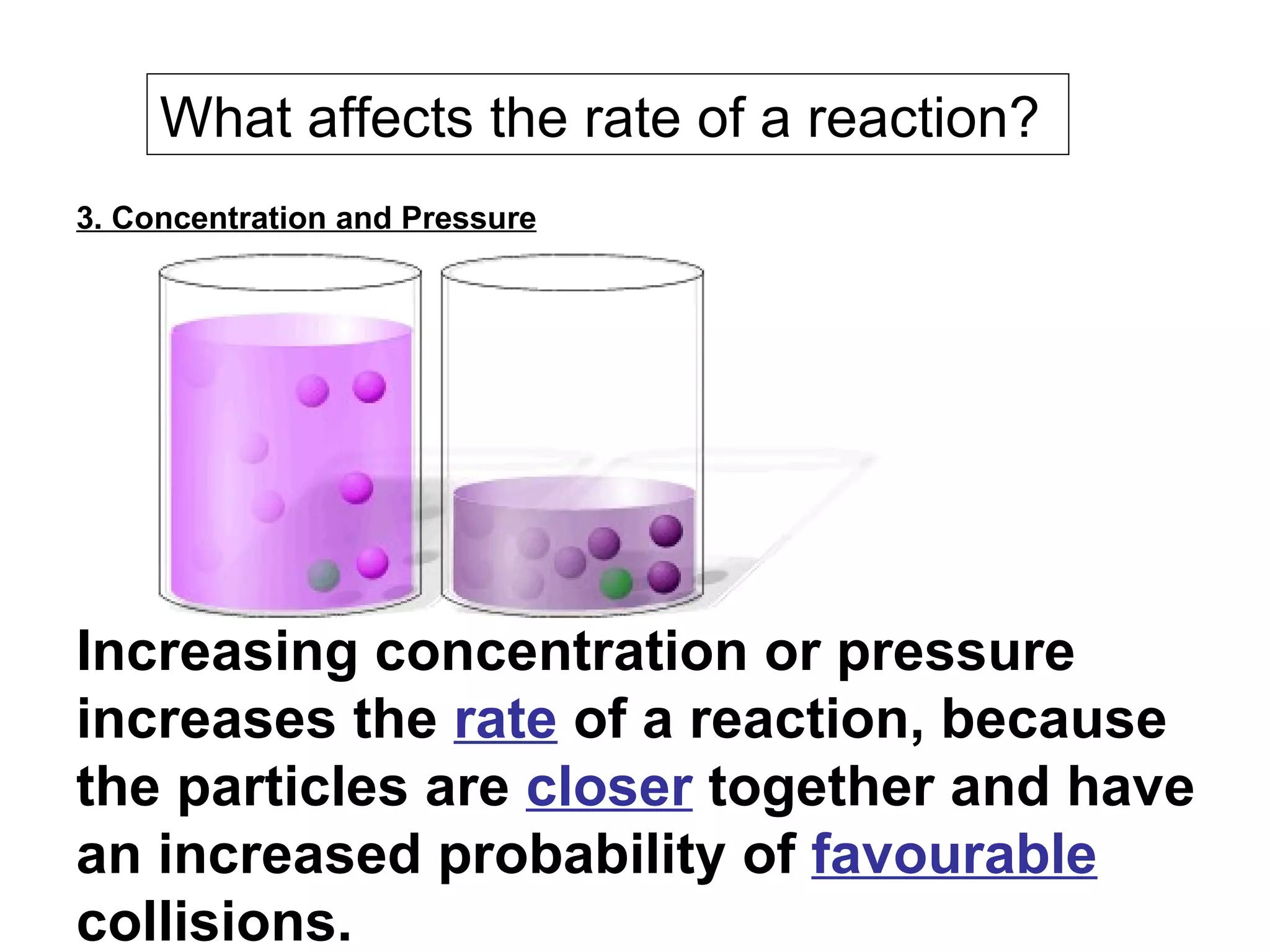
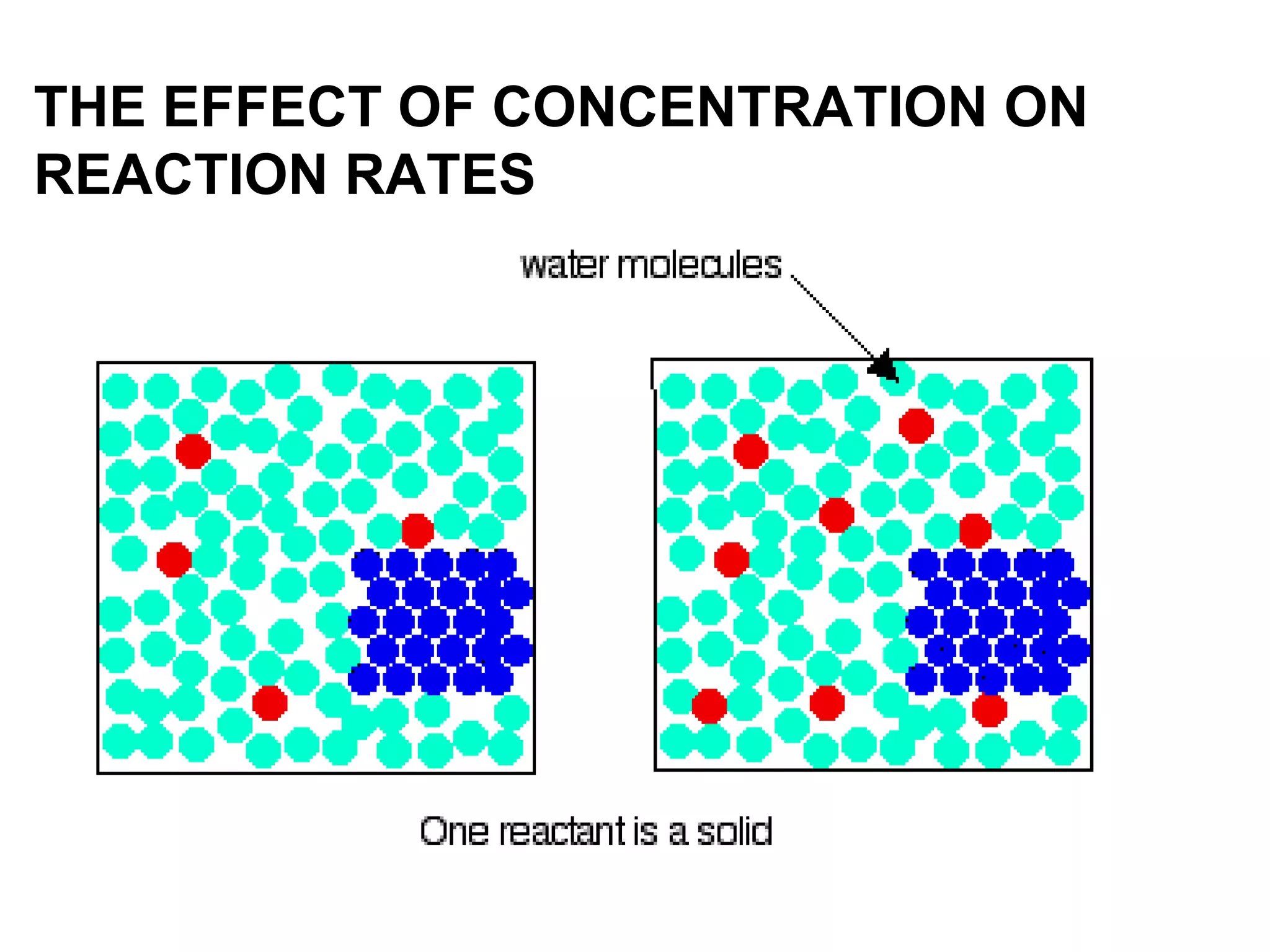
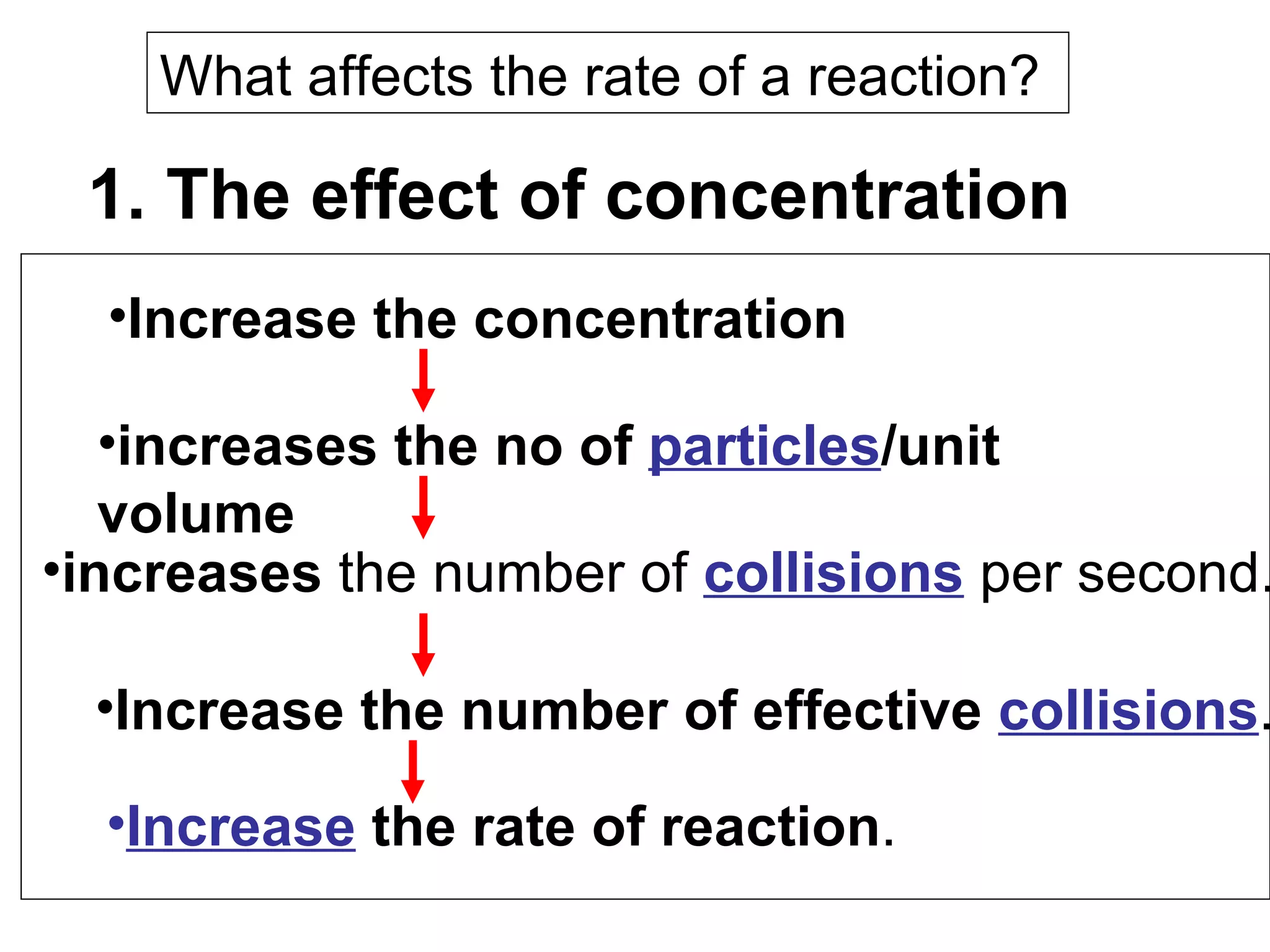
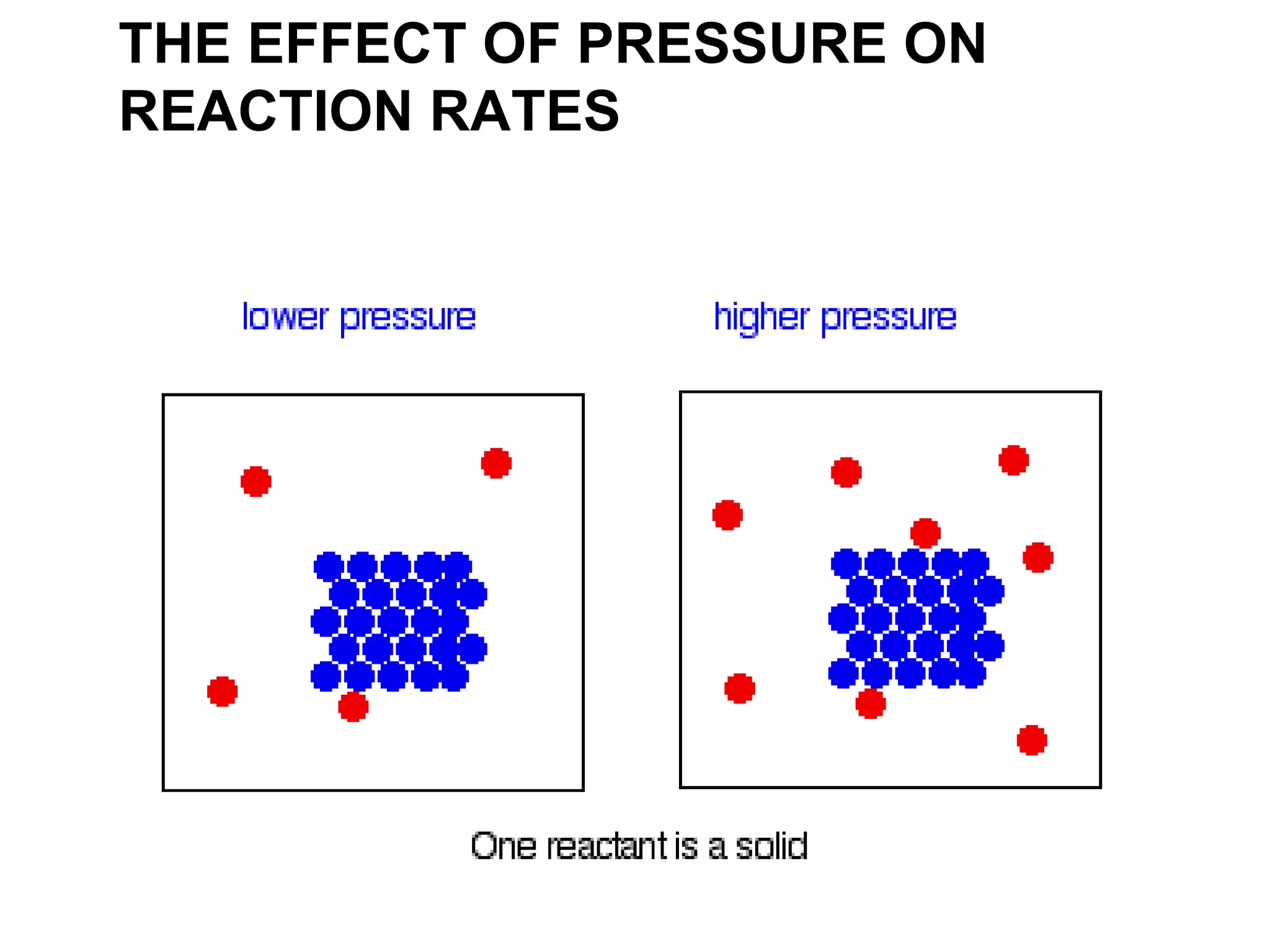
- Factors that affect reaction rates such as temperature, concentration, surface area, and the use of catalysts. Increased factors lead to more collisions and faster reactions.
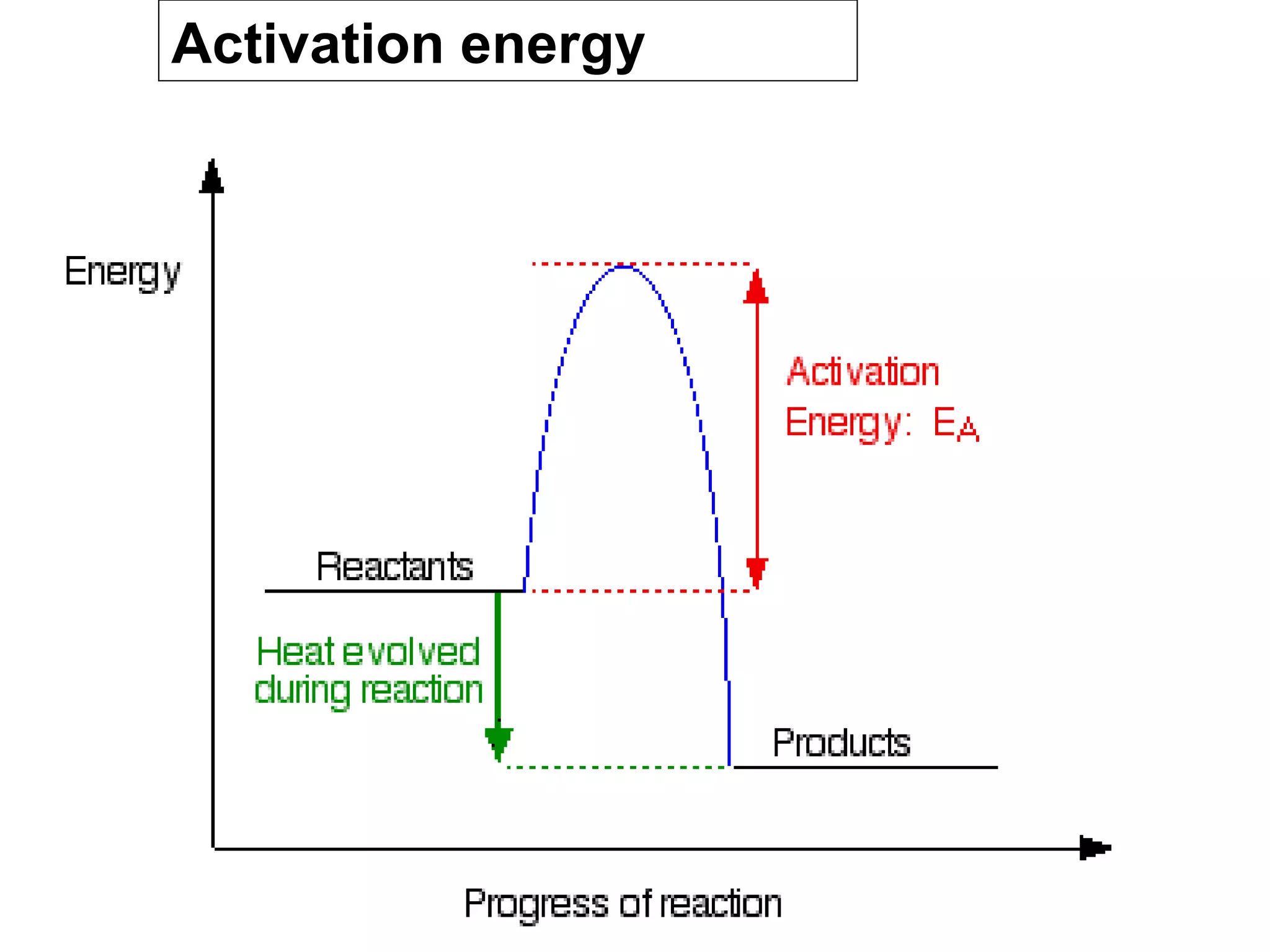
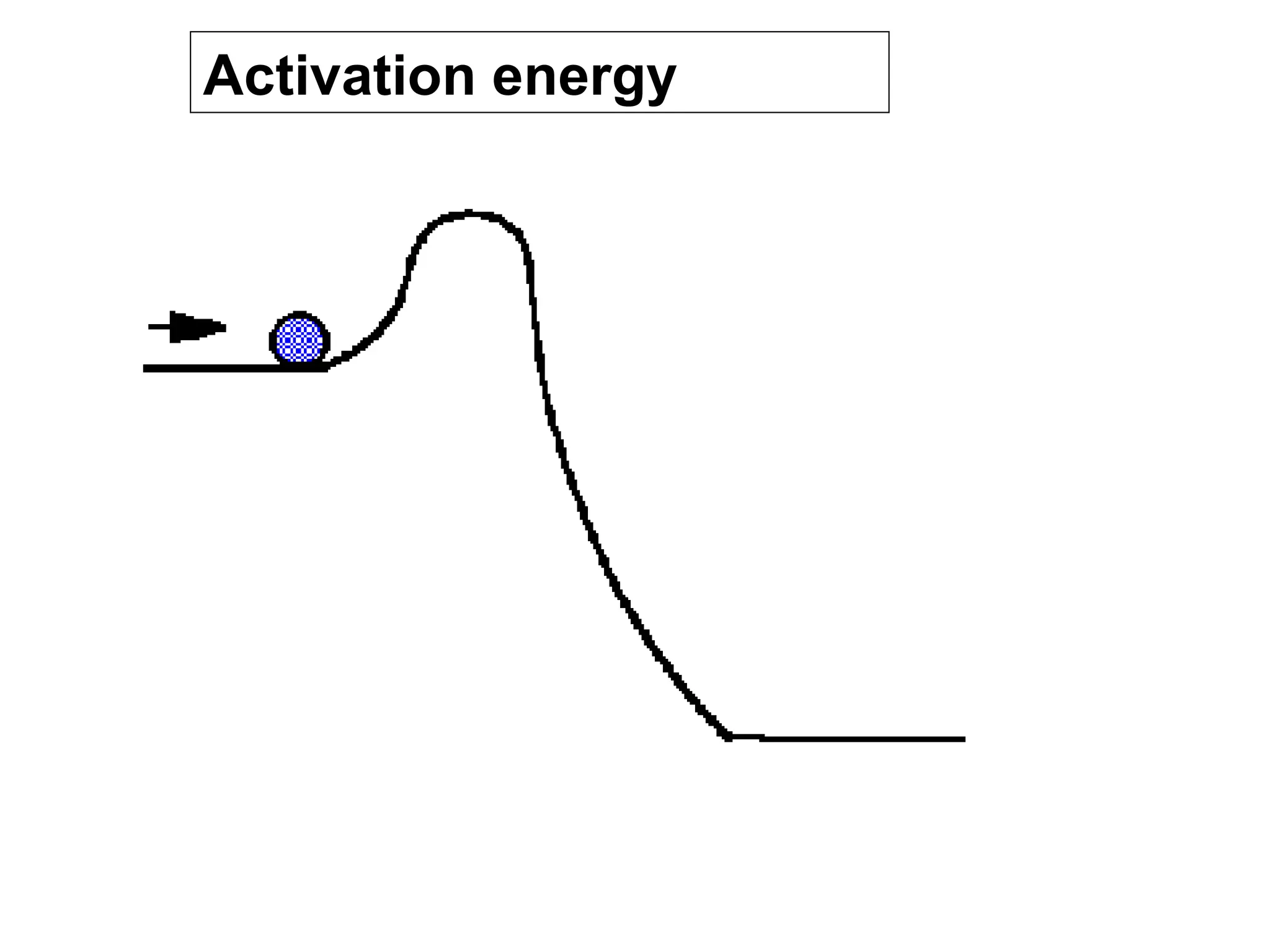
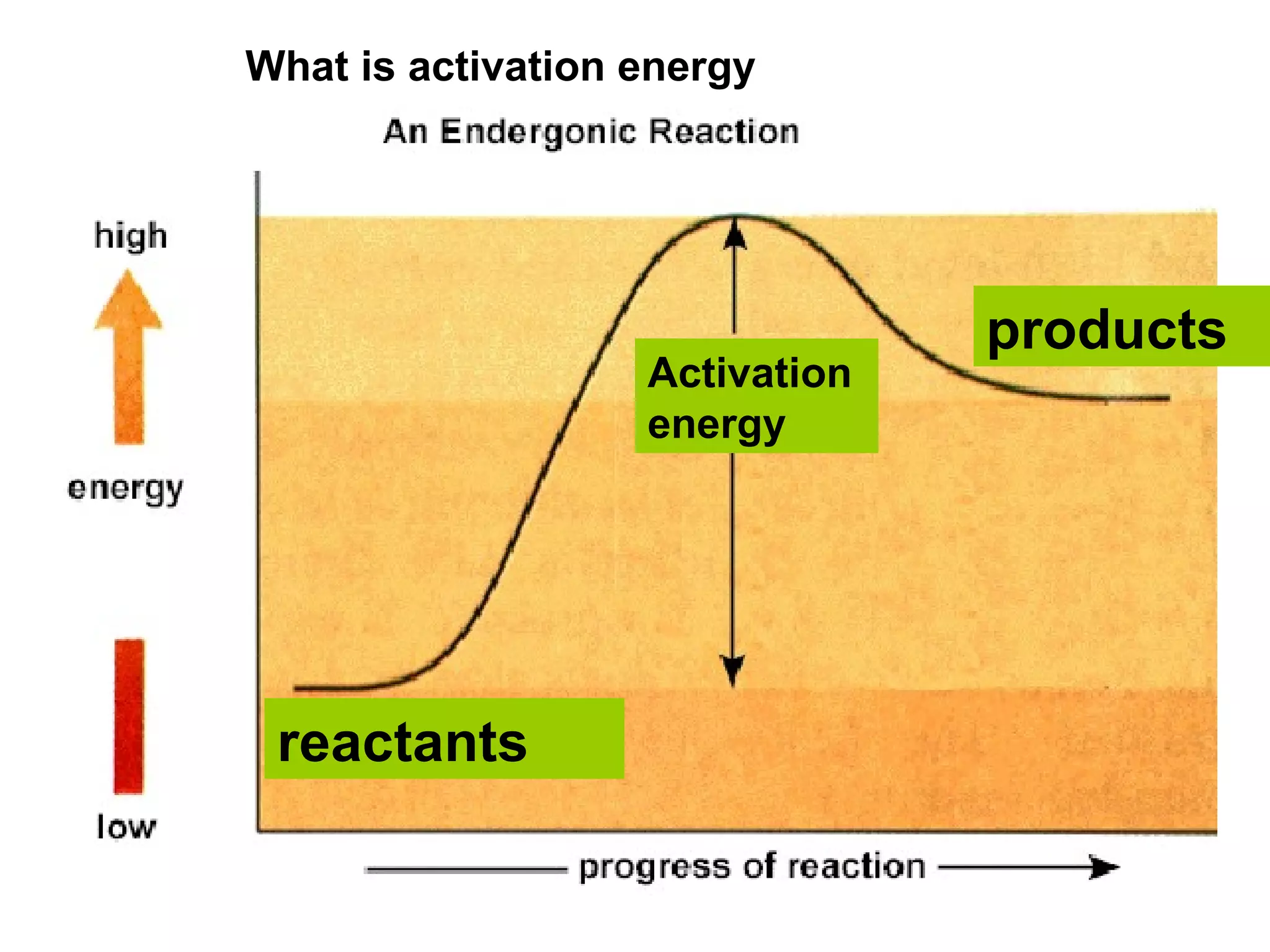
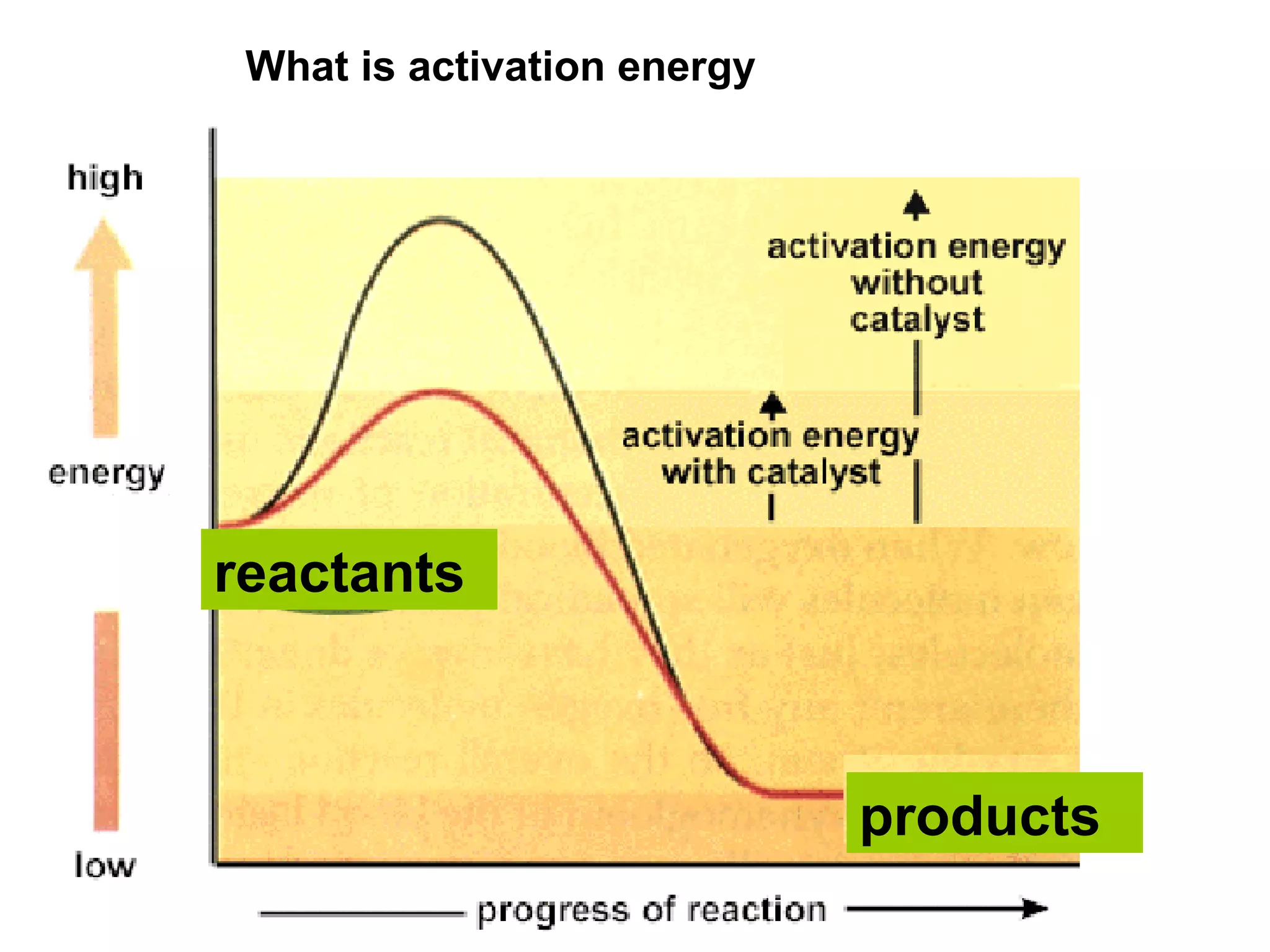
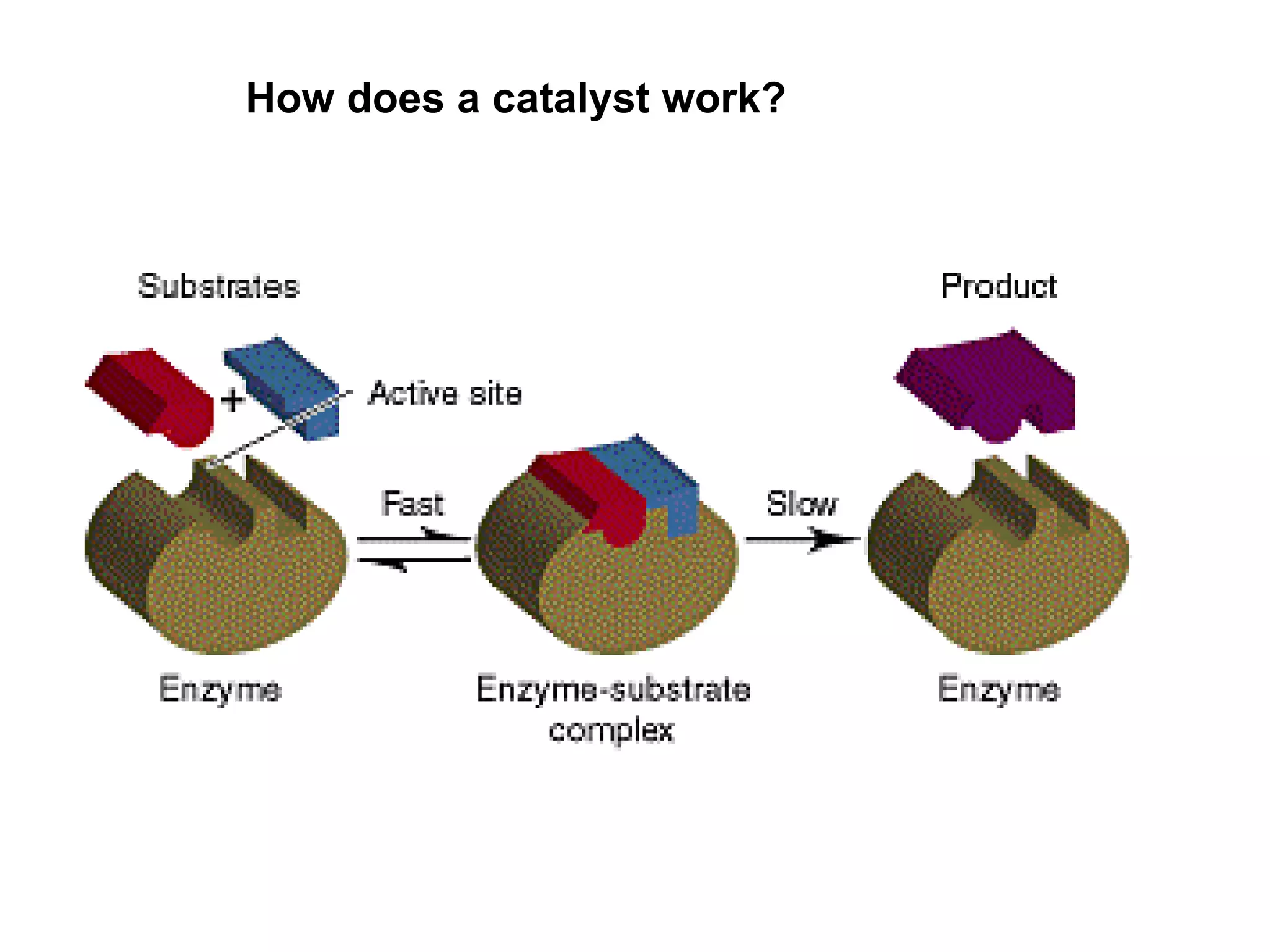
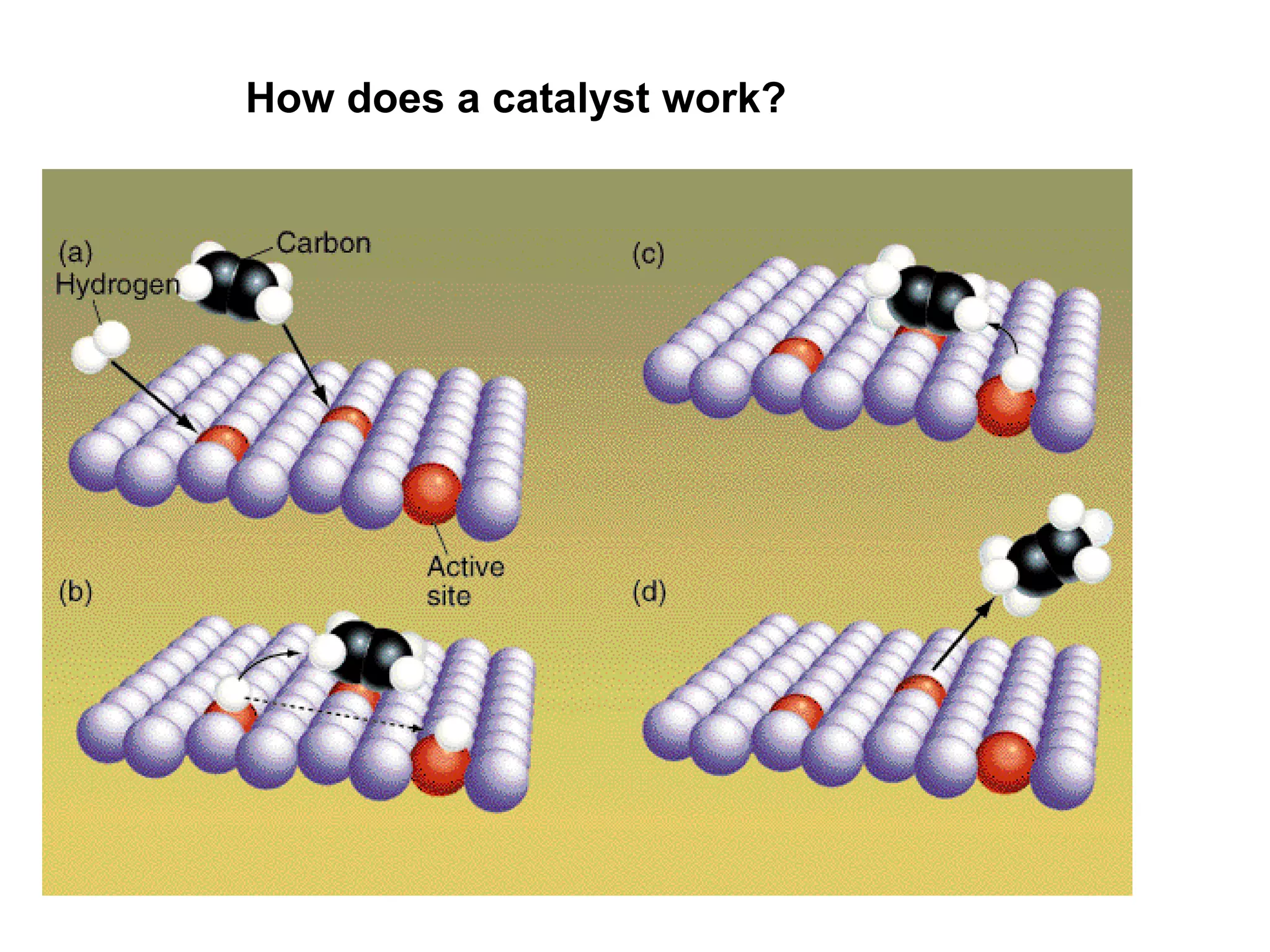
- Activation energy which is the minimum energy needed for molecules to react. Catalysts provide an alternative reaction pathway with lower activation energy.
- Energy level diagrams which show reactants at a higher energy level than products for exothermic reactions, and lower for endothermic reactions.