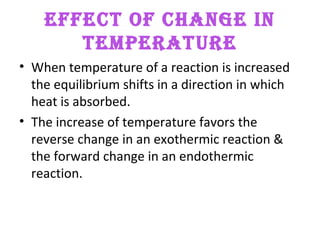
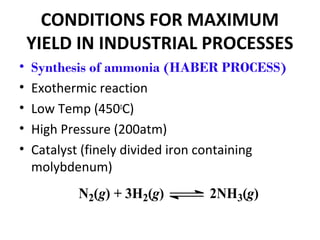
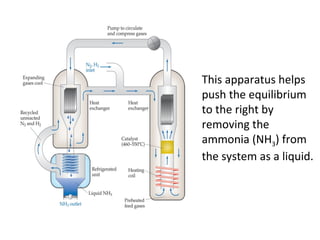
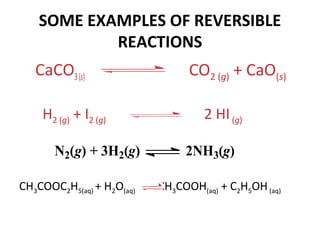
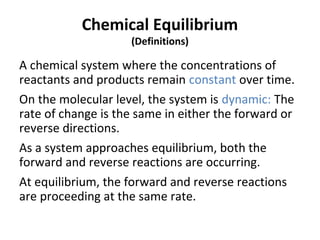
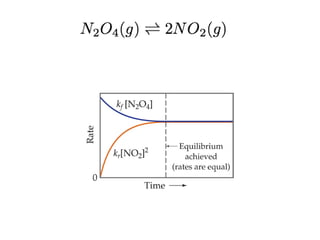
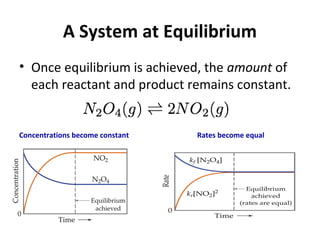
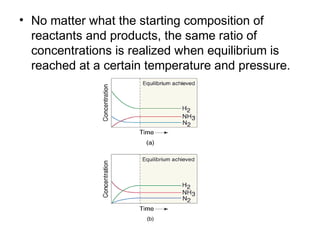
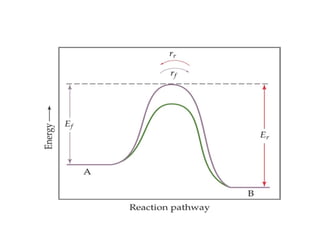
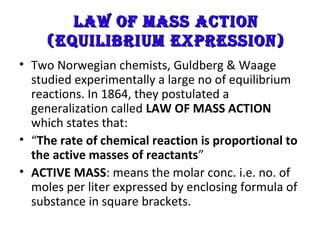
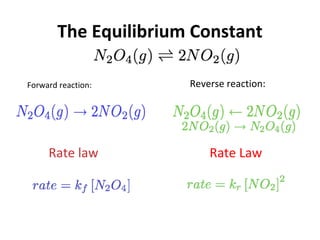
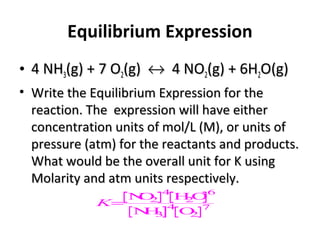
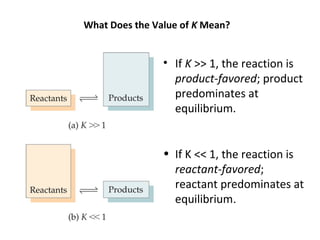
This document discusses the key concepts of chemical equilibrium. It defines reversible reactions as those that can proceed in both the forward and backward directions simultaneously. At equilibrium, the rates of the forward and reverse reactions are equal and the concentrations of reactants and products remain constant. Several examples of reversible reactions are provided. Characteristics of chemical equilibrium include the constancy of concentrations at equilibrium and the independence of the equilibrium constant from the initial concentrations. Le Chatelier's principle is introduced, which states that if a system at equilibrium experiences a change, it will shift its position to counteract that change. The effects of changing concentration, pressure, temperature, and adding a catalyst are described based on this principle. Industrial processes for maximizing yields of important chemicals








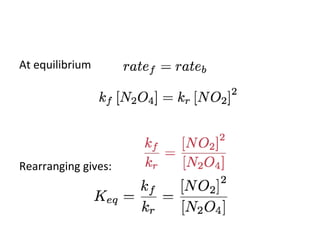
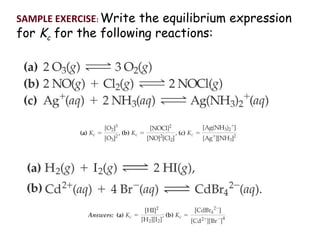
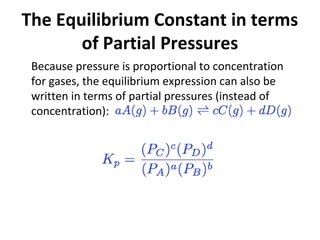
![Relationship between Kc and Kp
From the ideal gas law we know that:
Substituting P=[A]RT into the expression for Kp for
each substance, the relationship between Kc and Kp
becomes: Kp = Kc (RT)∆n](https://image.slidesharecdn.com/chemicalequilibrium-171025105826/85/Chemical-equilibrium-23-320.jpg)

![b
B
a
A
d
D
c
C
c
ba
dc
c
RT
P
RT
P
RT
P
RT
P
K
K
=
=
+↔+
ions...concentratfor thesubstitutewe
]B[]A[
]D[]C[
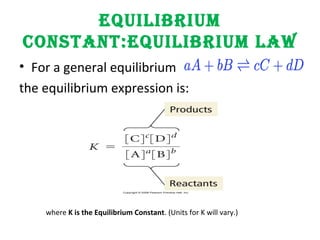
dD,cCbBaAgeneralFor the
b
B
a
A
d
D
c
C
c
ba
dc
c
RT
P
RT
P
RT
P
RT
P
K
K
=
=
+↔+
ions...concentratfor thesubstitutewe
]B[]A[
]D[]C[
dD,cCbBaAgeneralFor the
n
cp
n
pc
badc
pc
ba
b
B
a
A
dc
d
D
c
C
c
RTKK
RT
KK
RT
KK
RT
PP
RT
PP
K
∆
∆
+−+
+
+
=
=
=
=
)(
1
1
1
1
)(
n
cp
n
pc
badc
pc
ba
b
B
a
A
dc
d
D
c
C
c
RTKK
RT
KK
RT
KK
RT
PP
RT
PP
K
∆
∆
+−+
+
+
=
=
=
=
)(
1
1
1
1
)(
n
cp
n
pc
badc
pc
ba
b
B
a
A
dc
d
D
c
C
c
RTKK
RT
KK
RT
KK
RT
PP
RT
PP
K
∆
∆
+−+
+
+
=
=
=
=
)(
1
1
1
1
)(](https://image.slidesharecdn.com/chemicalequilibrium-171025105826/85/Chemical-equilibrium-25-320.jpg)
![PROBLEM
• For the reaction 2SO3(g) 2SO2(g) + O2(g), we
can write two equilibrium expressions
• [SO2]= 0.27 mol/L [O2] = 0.40mol/L [SO3] =
0.33mol/L, calculate the value of Kc?
2
SO
O
2
SO
2
3
2
2
2
3
22
or
]SO[
]O[]SO[
P
PP
KK pc ==](https://image.slidesharecdn.com/chemicalequilibrium-171025105826/85/Chemical-equilibrium-27-320.jpg)
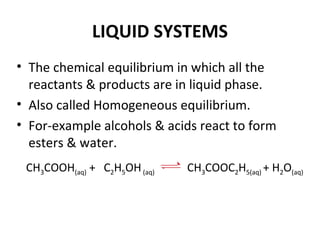
![HETEROGENEOUS EQUILIBRIUM
•If one or more reactants or products are in aIf one or more reactants or products are in a
different phase, the equilibrium isdifferent phase, the equilibrium is
heterogeneous.heterogeneous.
The conc. Of pure solids & liquids are notThe conc. Of pure solids & liquids are not
included in equilibrium constant expression asincluded in equilibrium constant expression as
the conc of pure solid/liquid is fixed & cannotthe conc of pure solid/liquid is fixed & cannot
vary. Kvary. Kcc= [CO= [CO22]]
CaCO3 (s) CO2 (g) + CaO(s)](https://image.slidesharecdn.com/chemicalequilibrium-171025105826/85/Chemical-equilibrium-29-320.jpg)