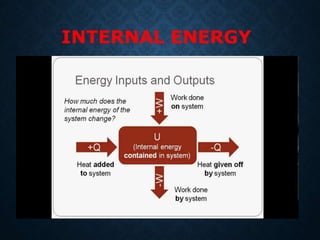
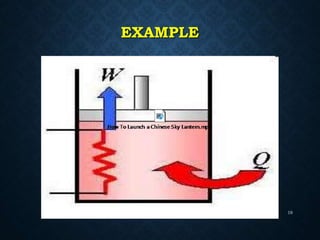
This document provides an overview of key concepts in thermodynamics including heat, work, internal energy, and the first law of thermodynamics. It defines heat as the transfer of energy between objects due to temperature difference and work as the energy transferred when an object is moved against a force. The first law of thermodynamics states that the total energy within an isolated system remains constant; energy can be converted between heat and work but not created or destroyed. It also explains how to determine the sign of heat and work transfers based on whether energy is entering or leaving the system and how to calculate changes in internal energy using the first law of thermodynamics.