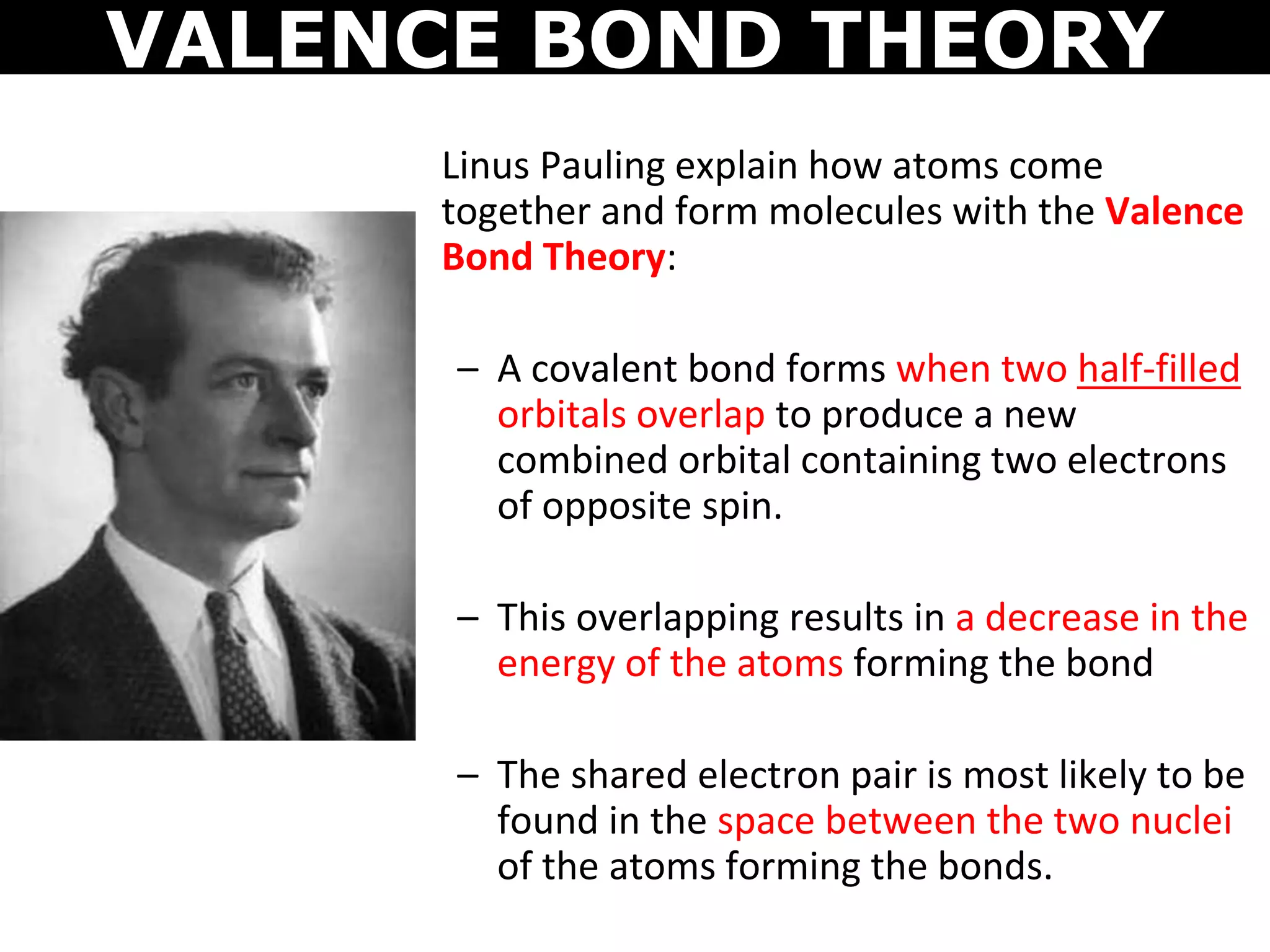
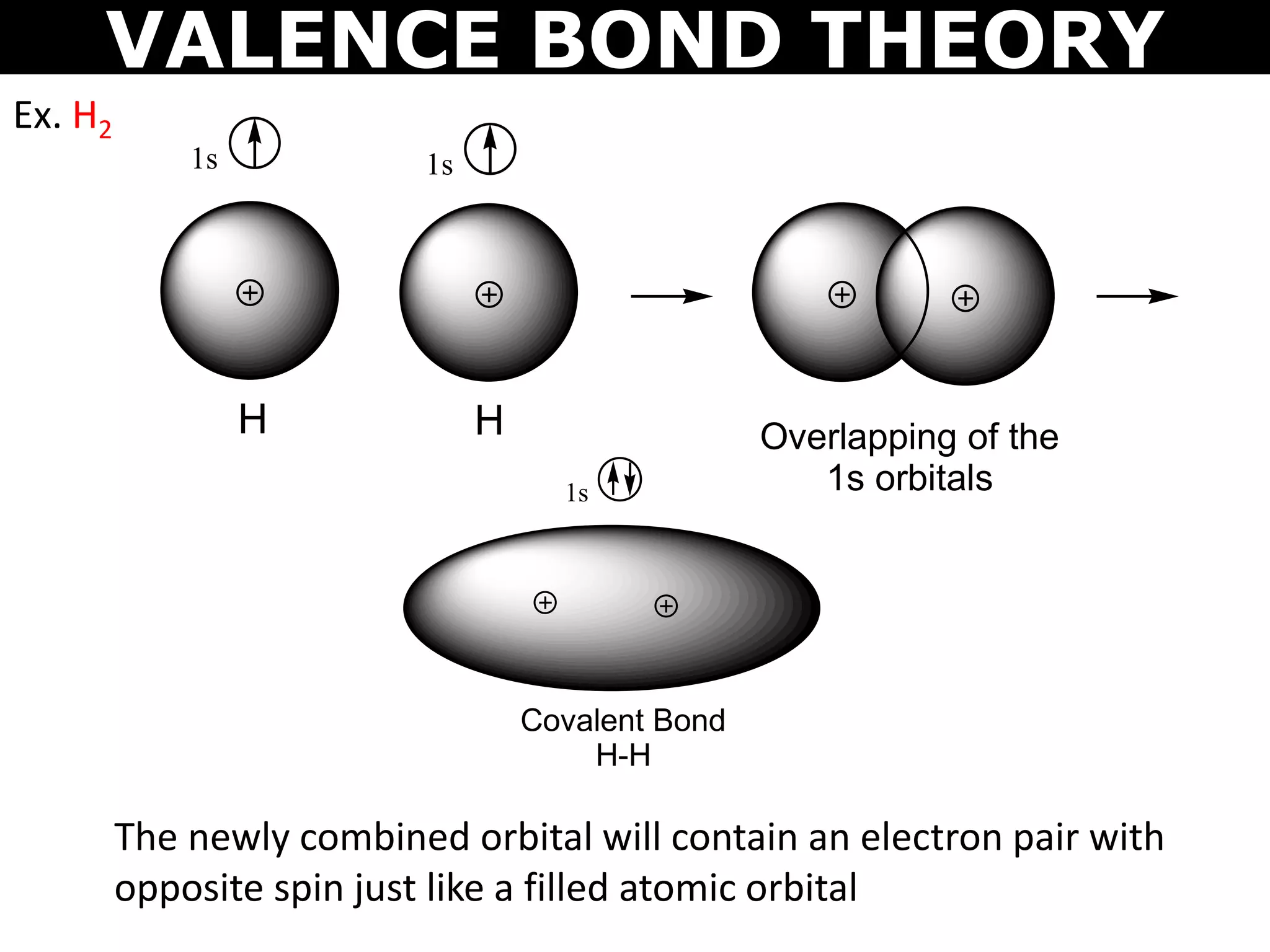
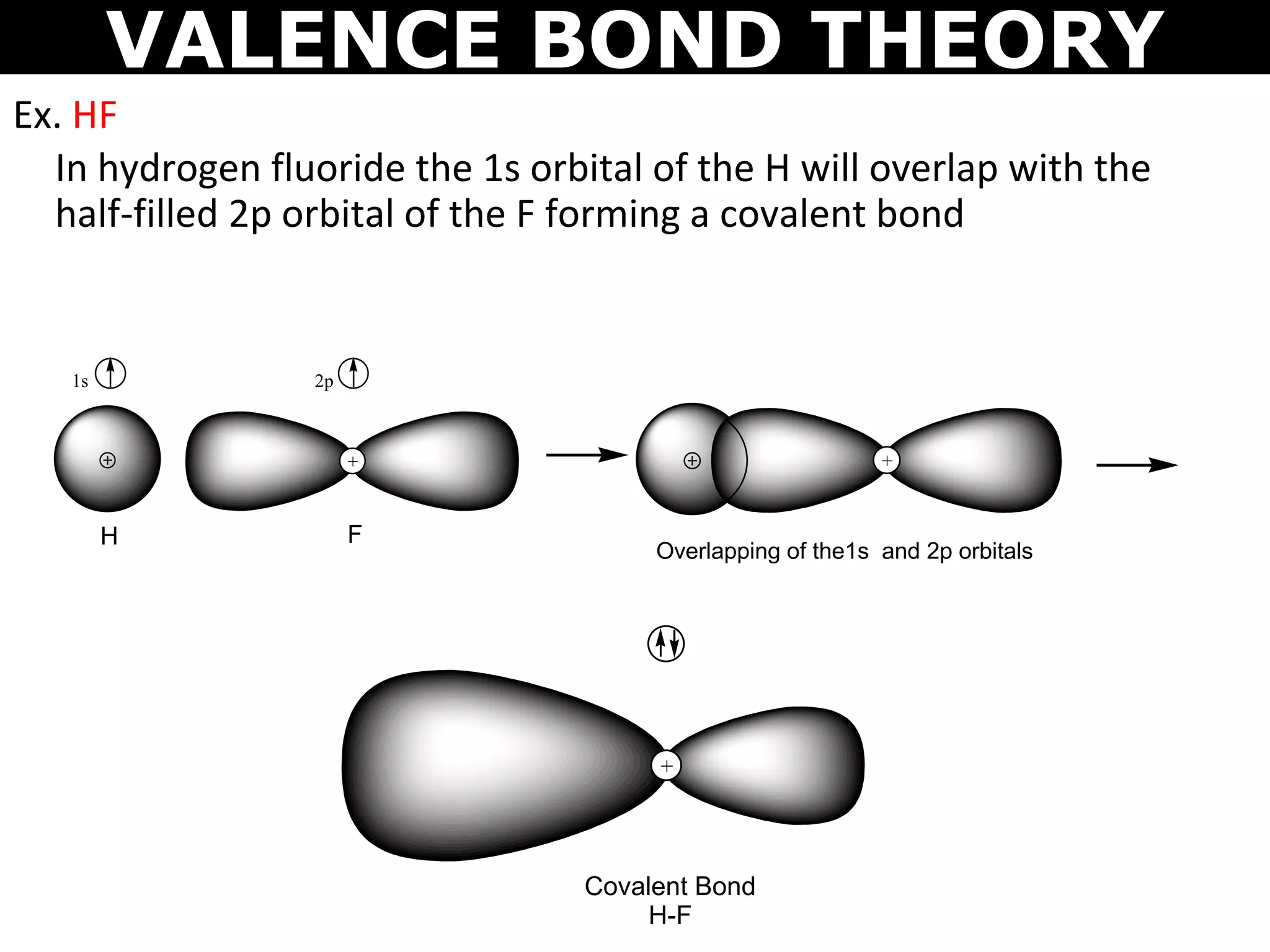
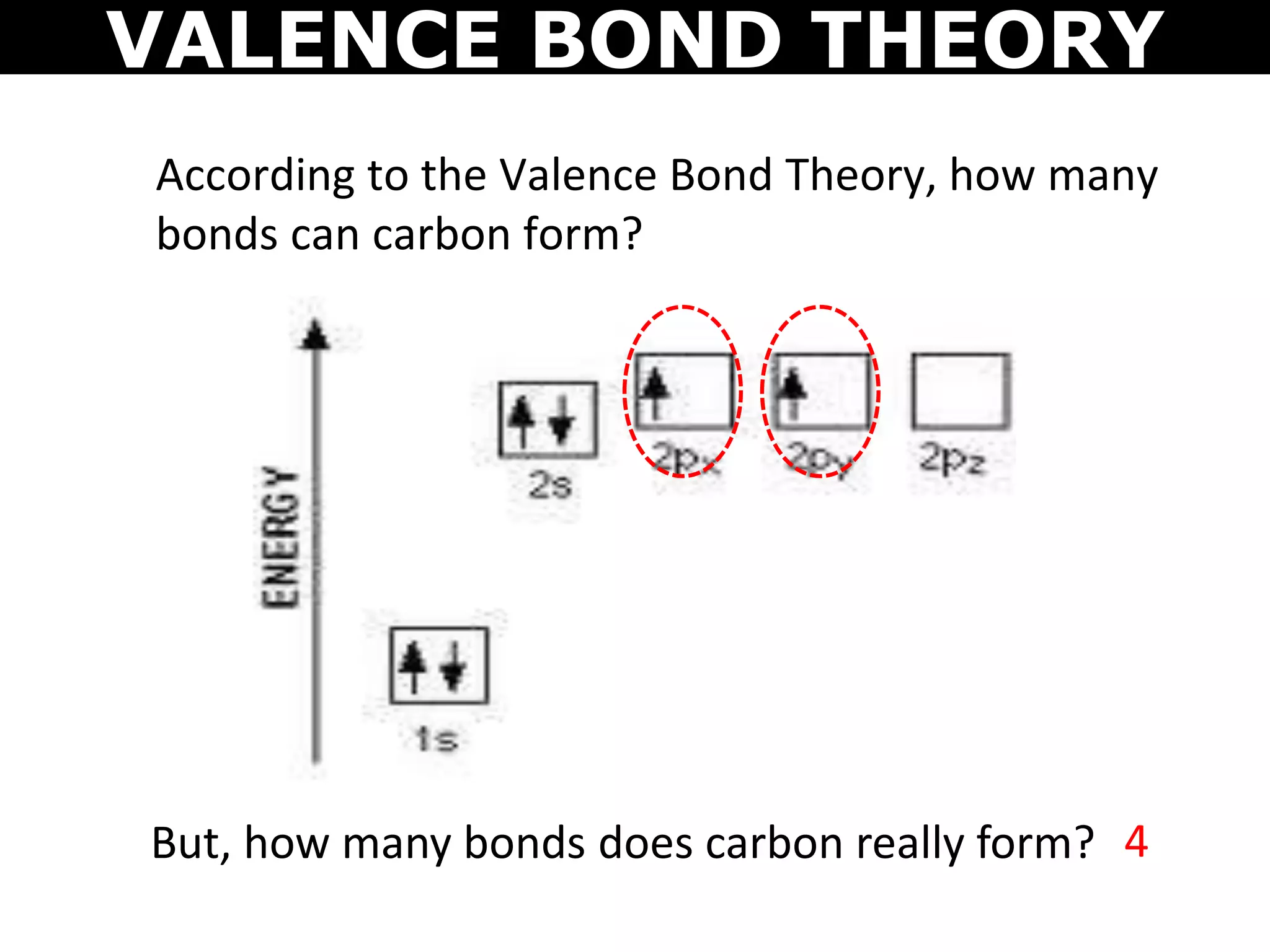
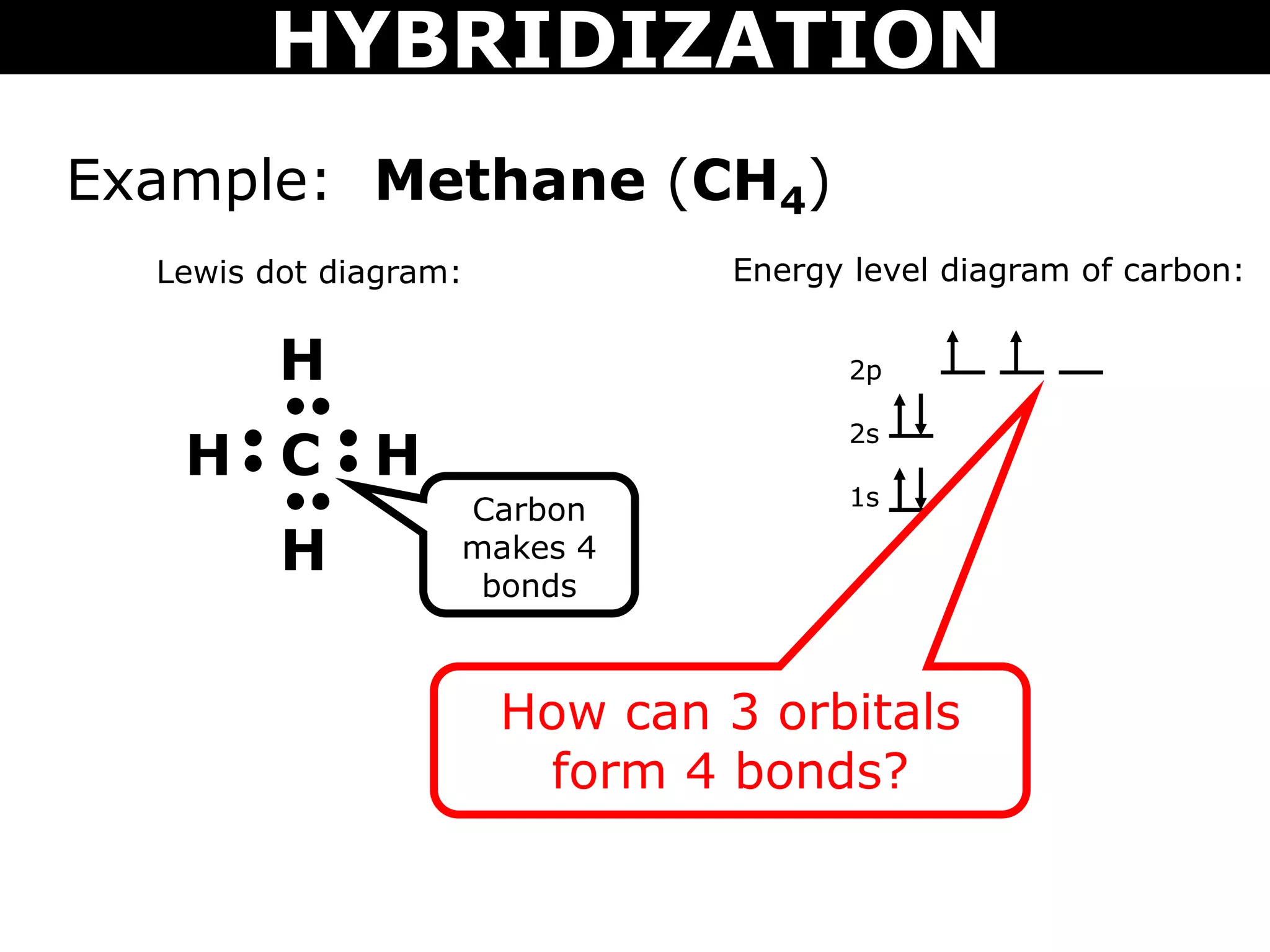
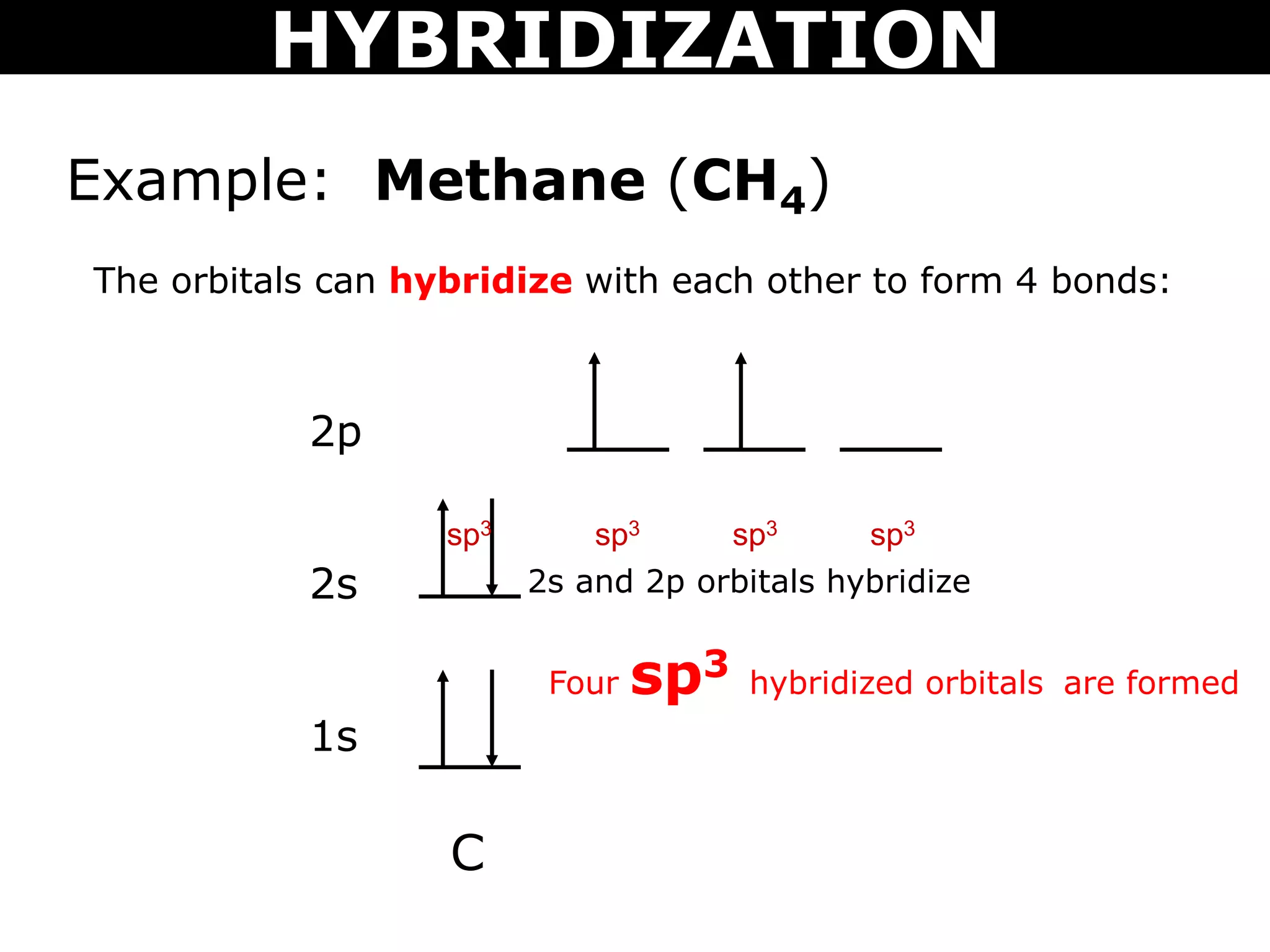
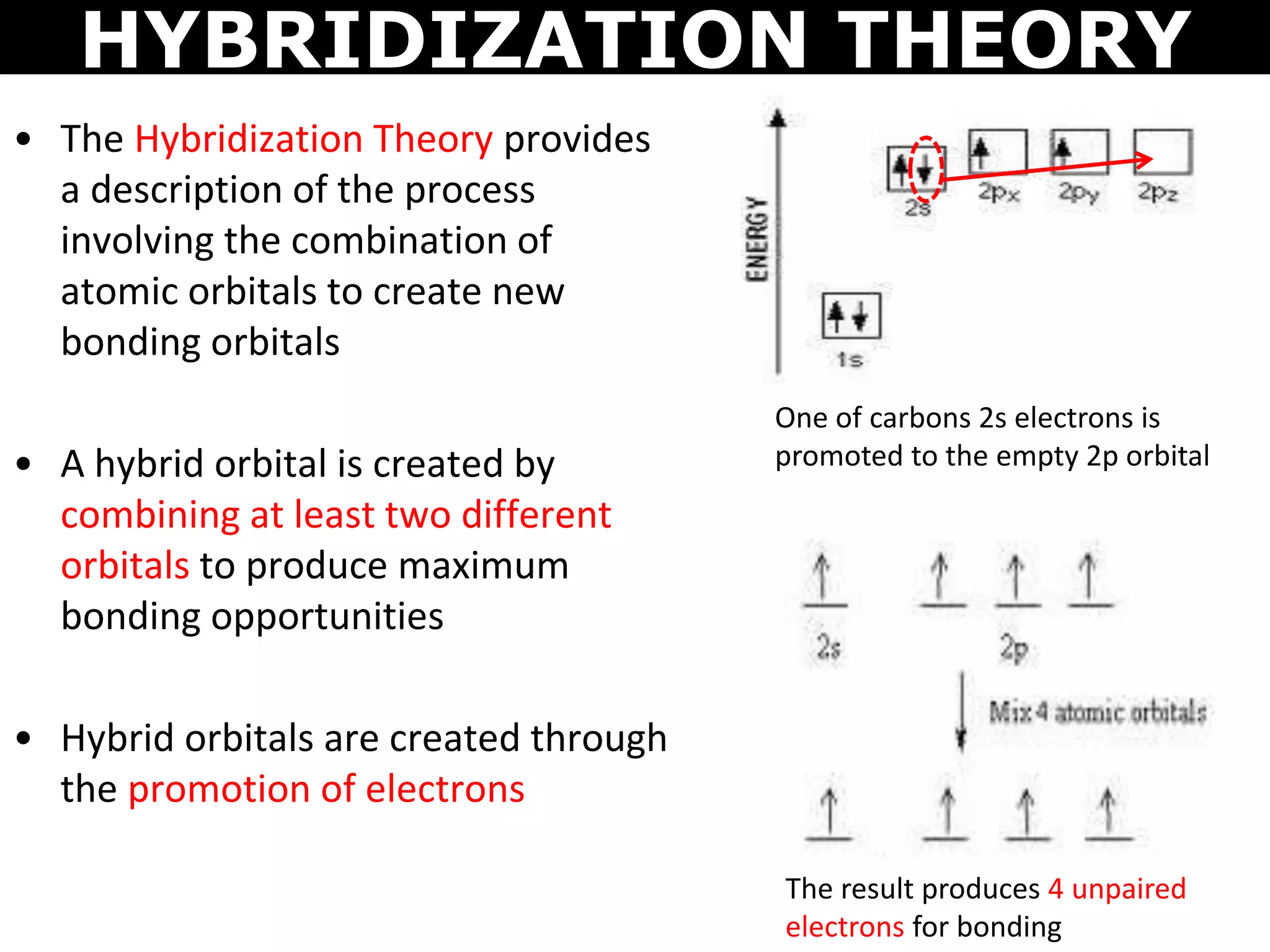
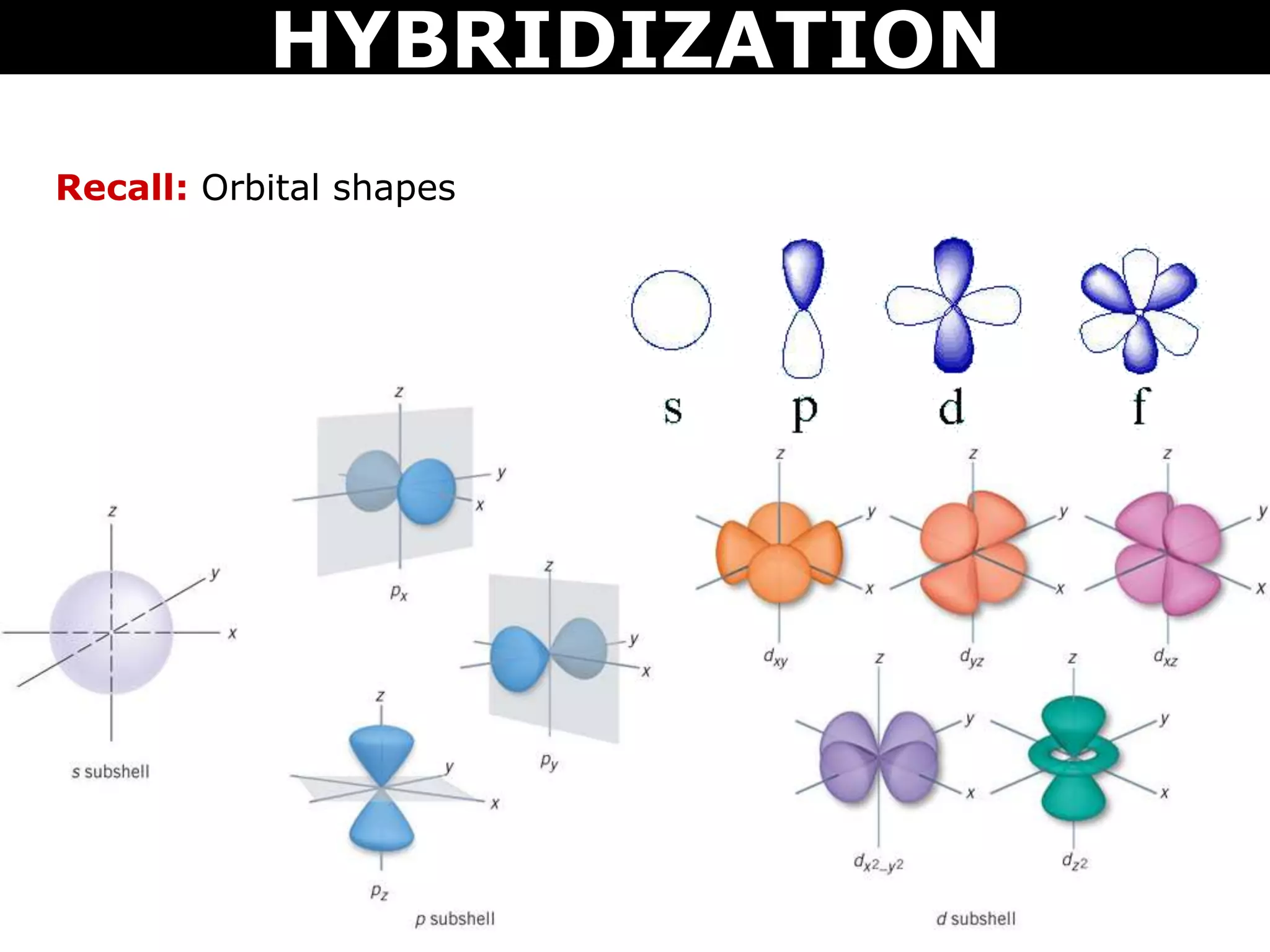
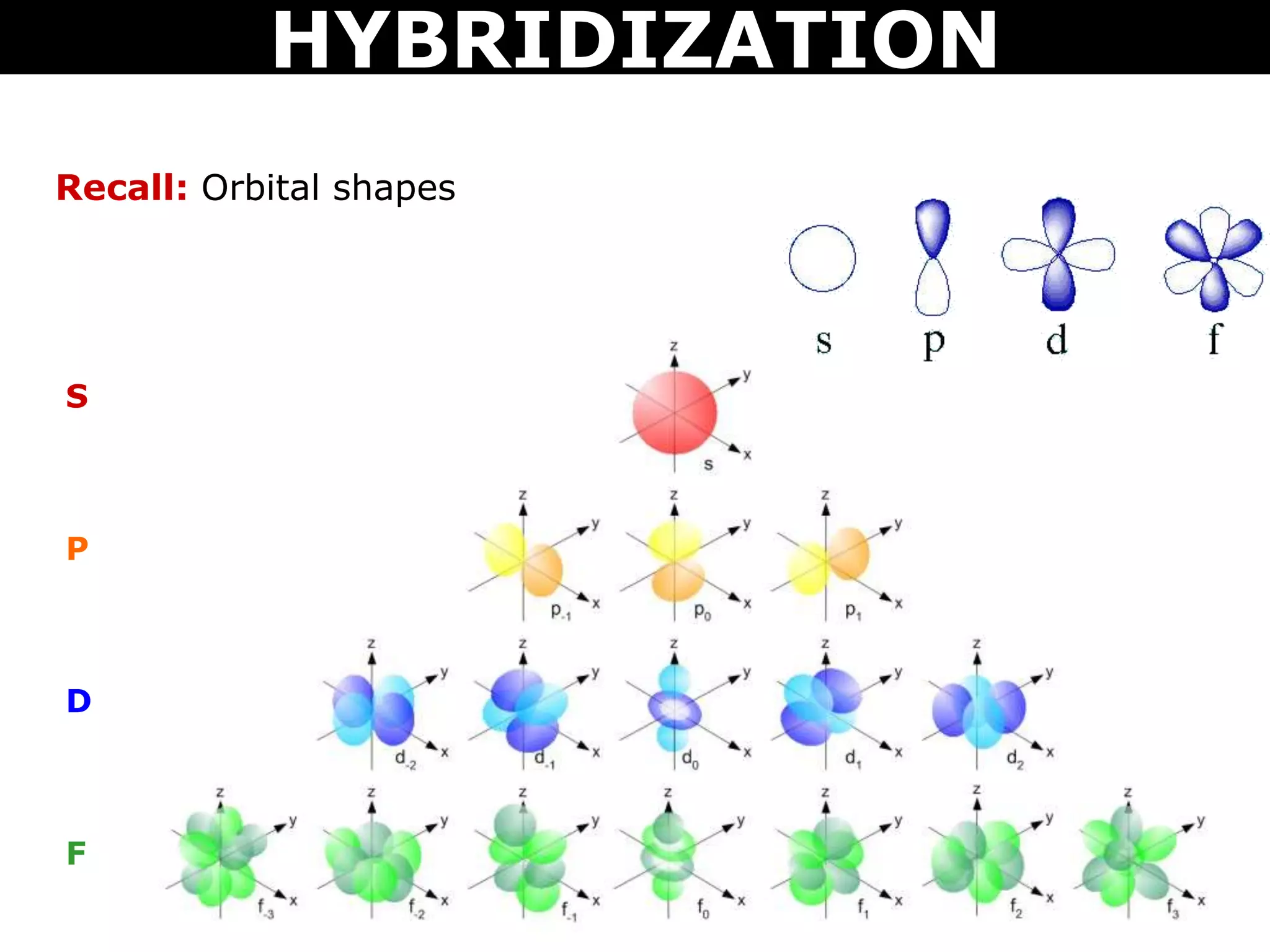
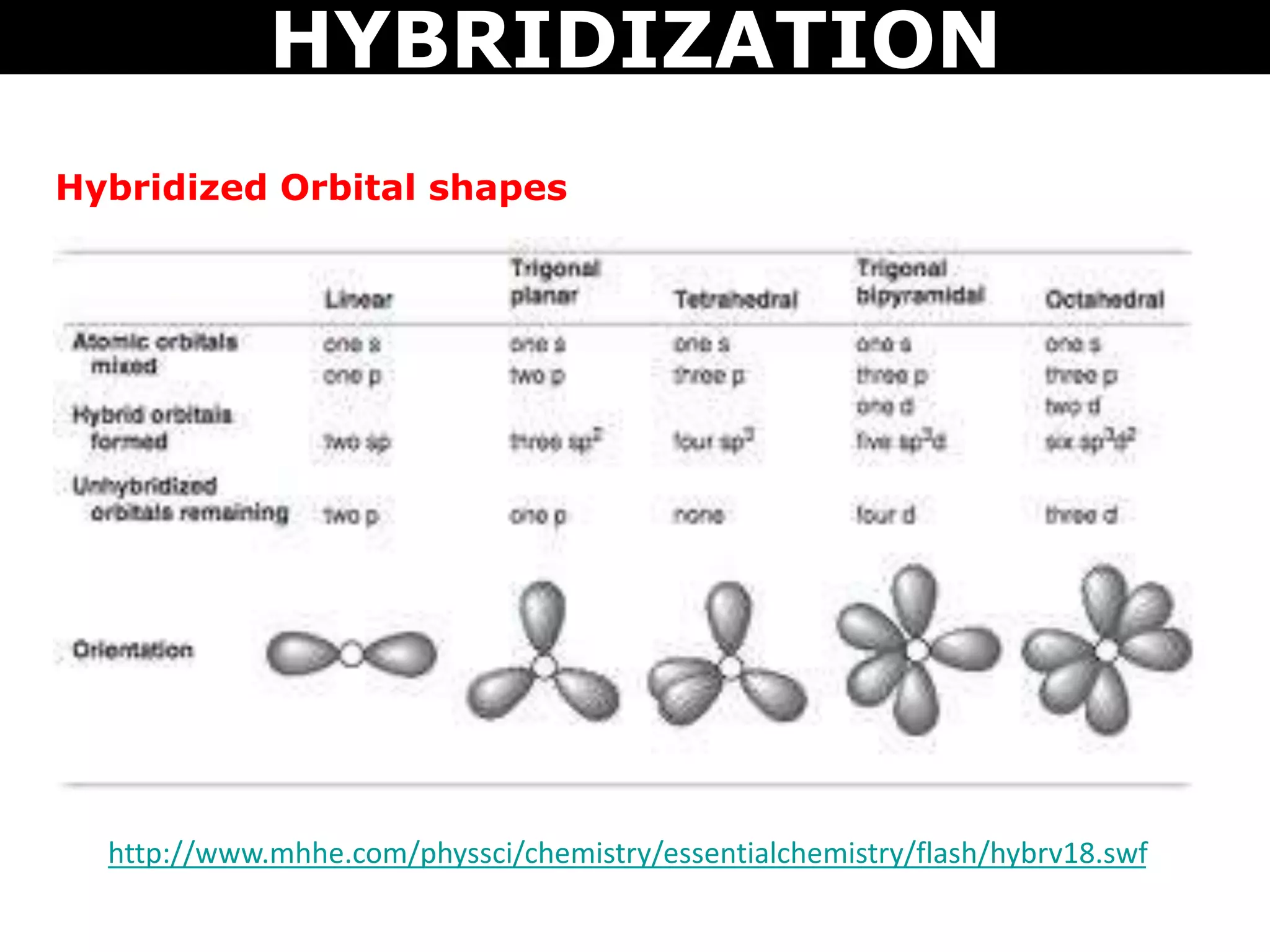
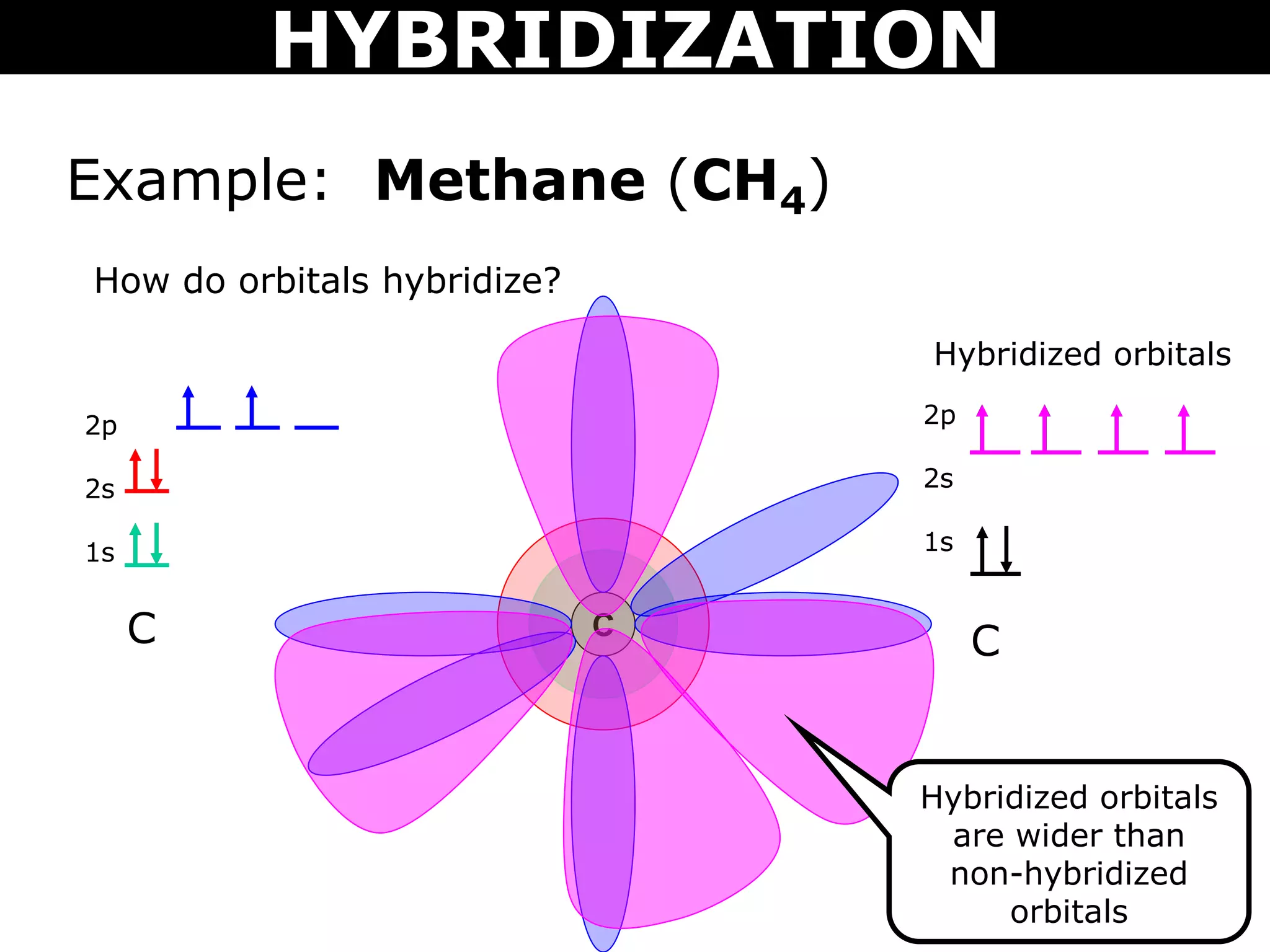
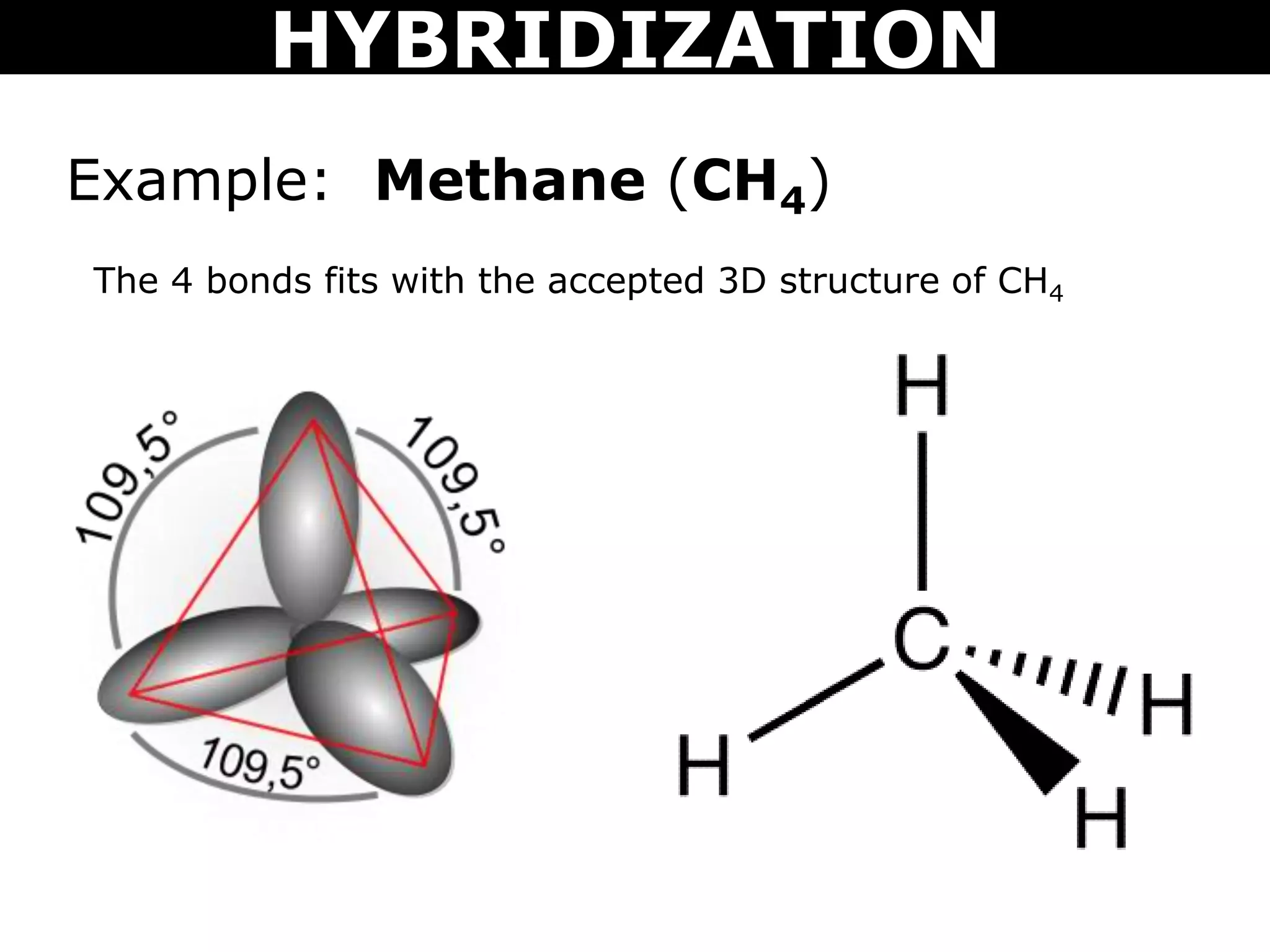
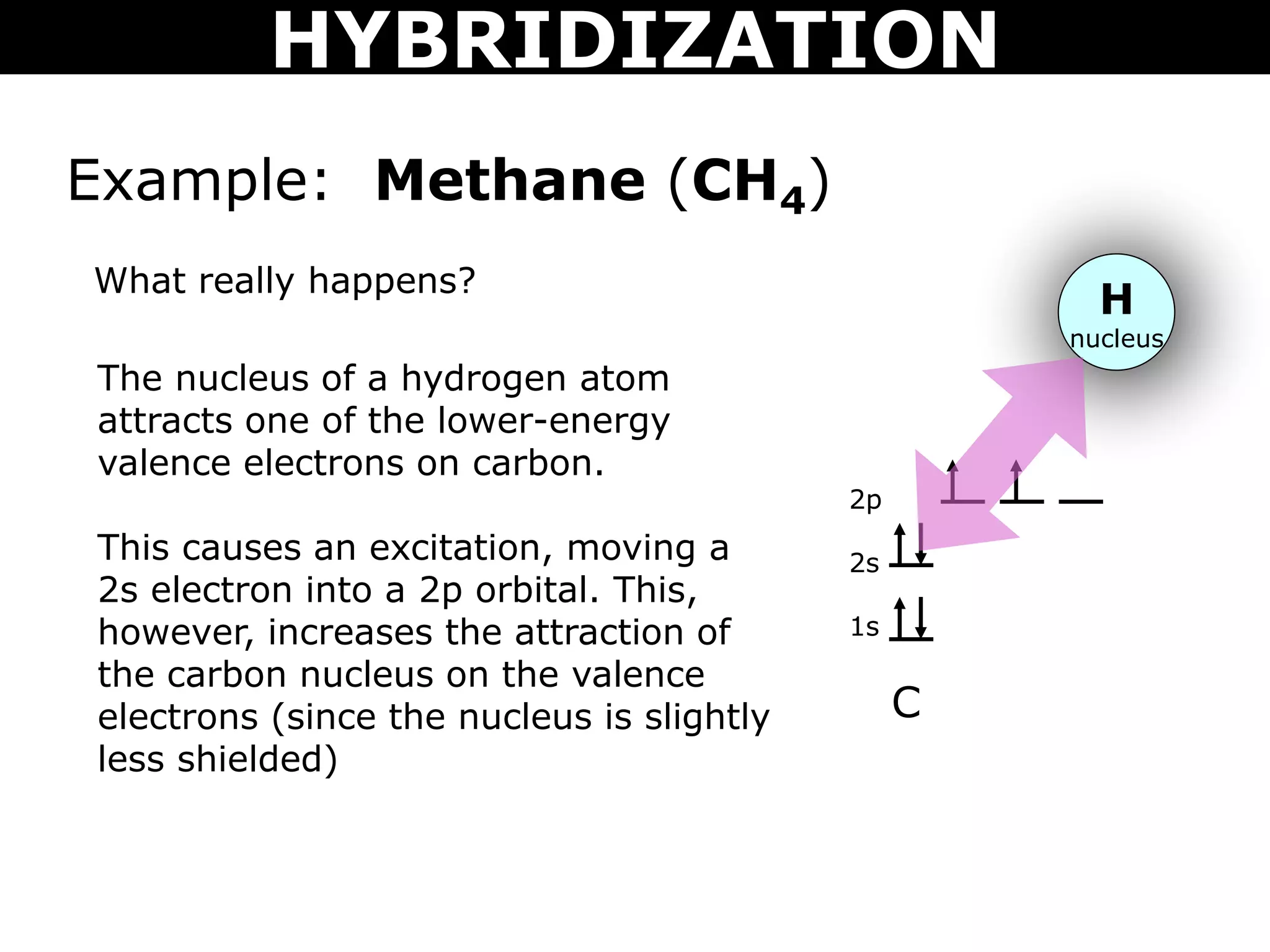
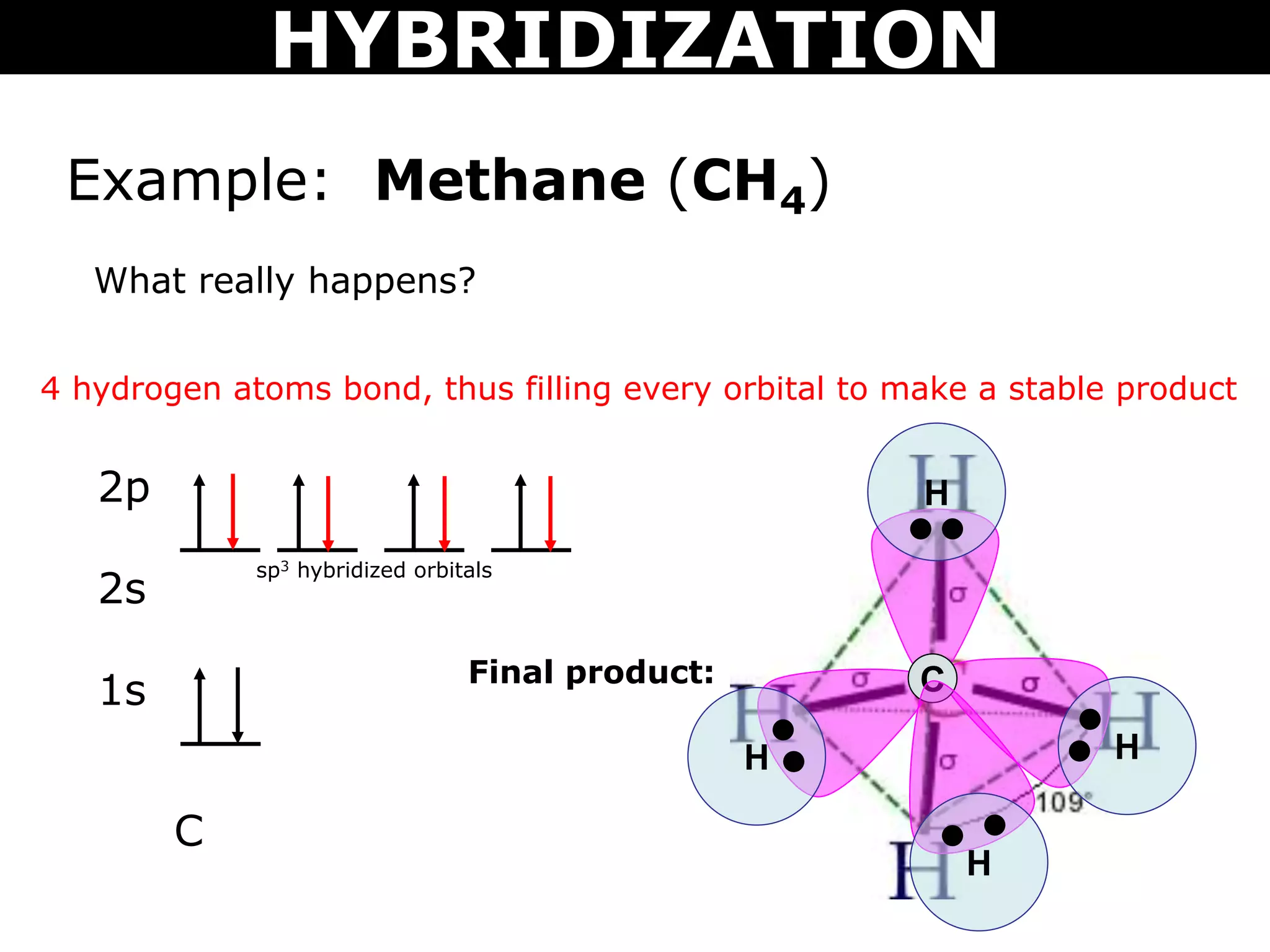
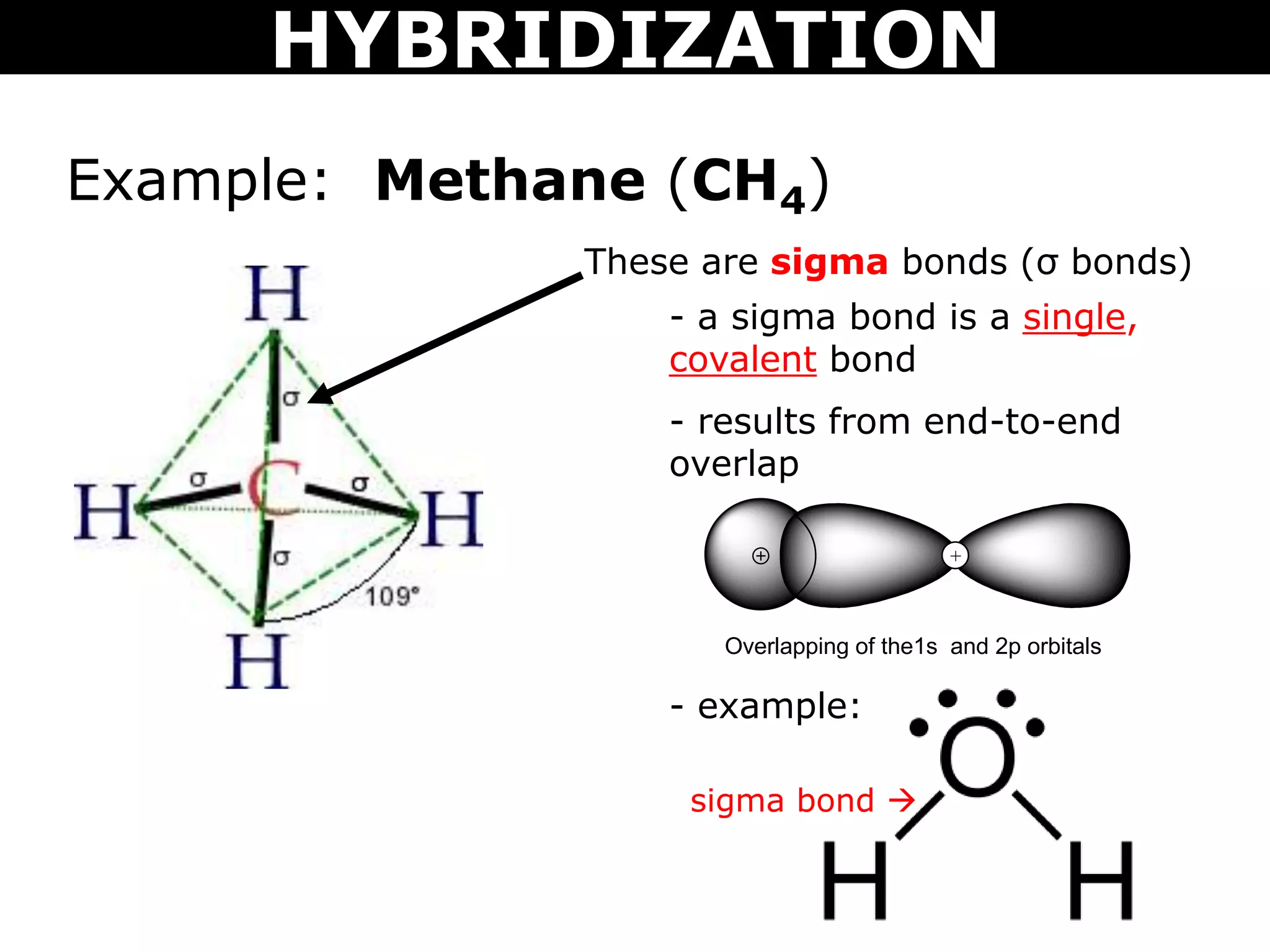
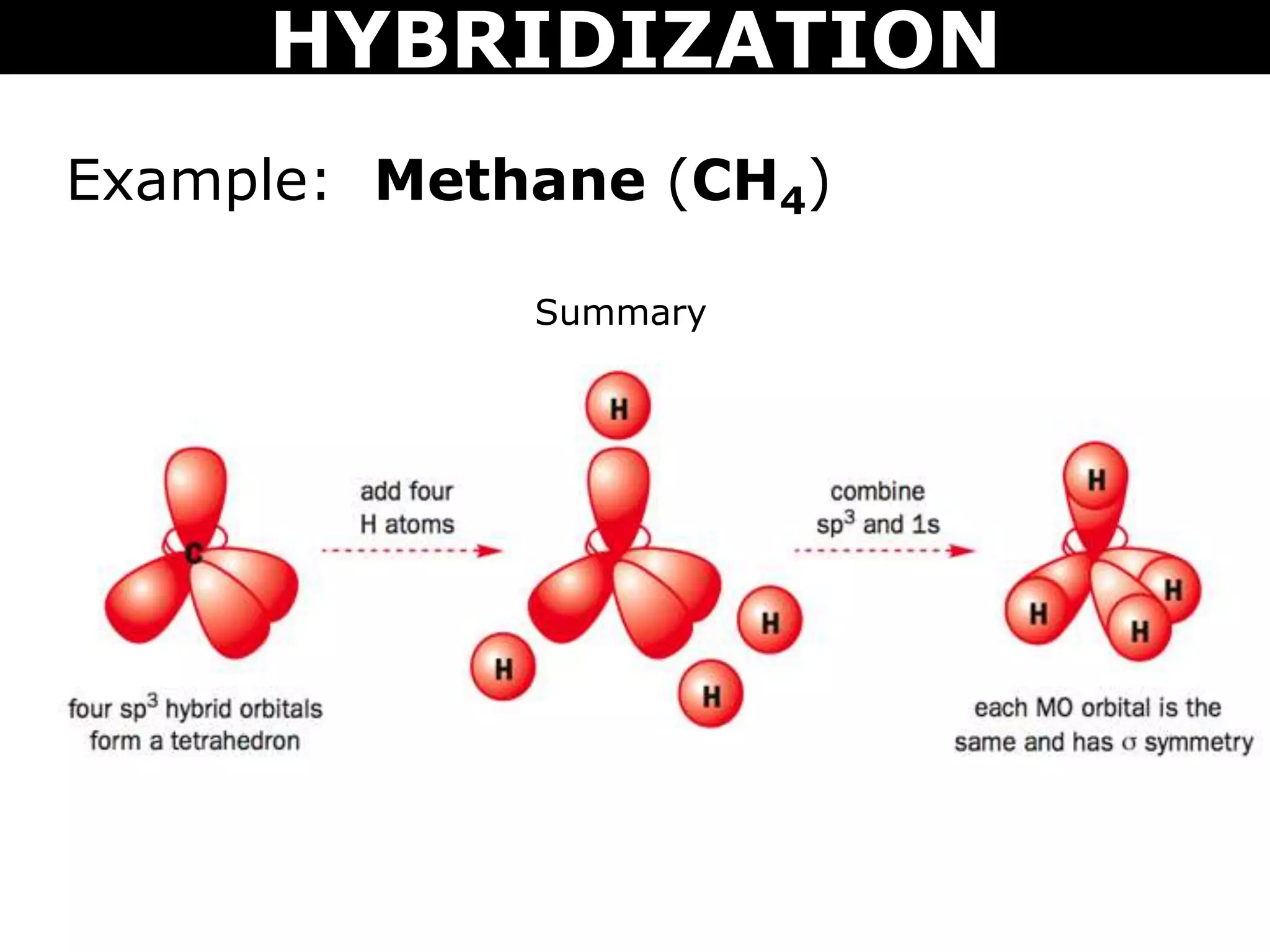
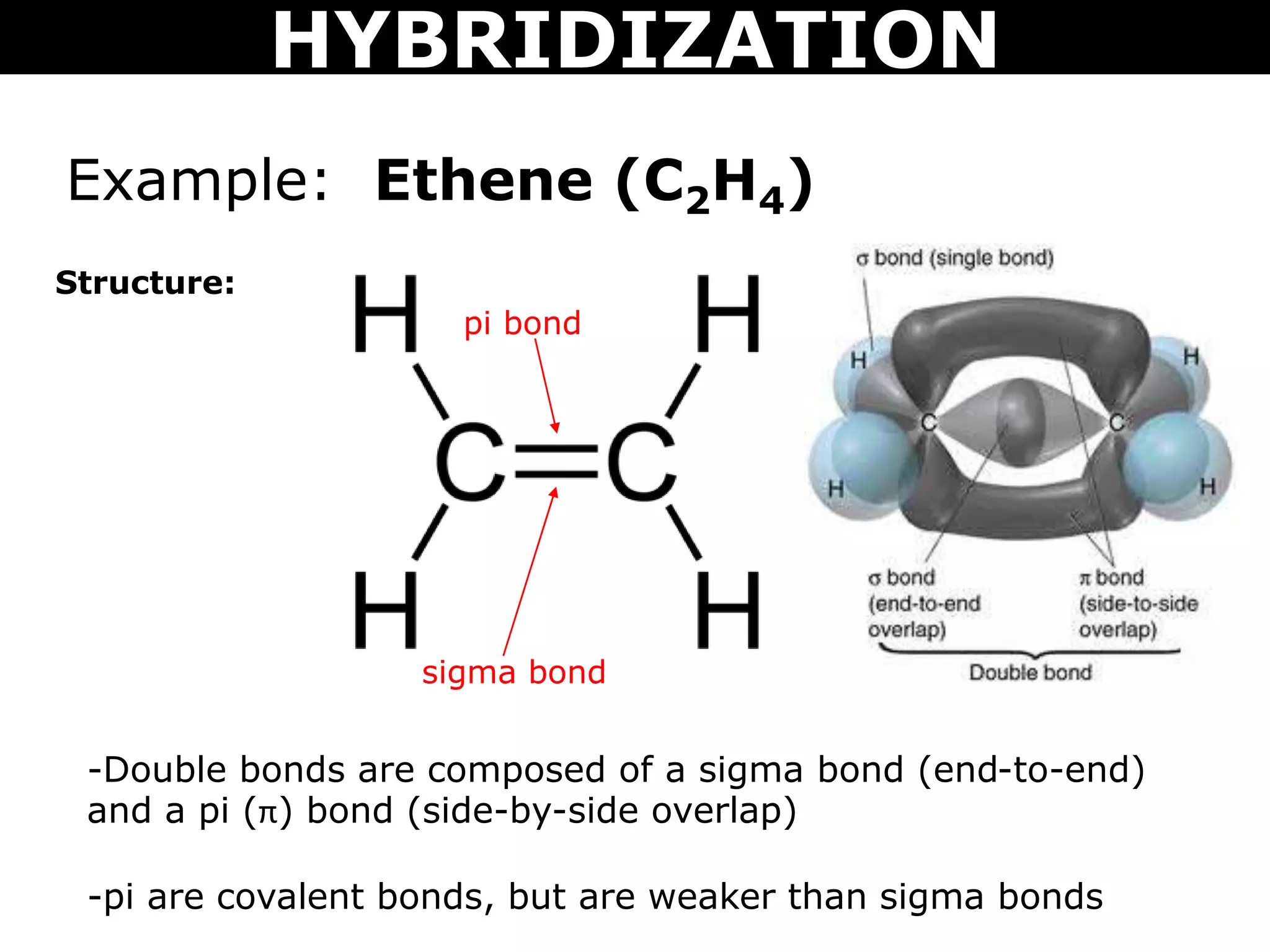
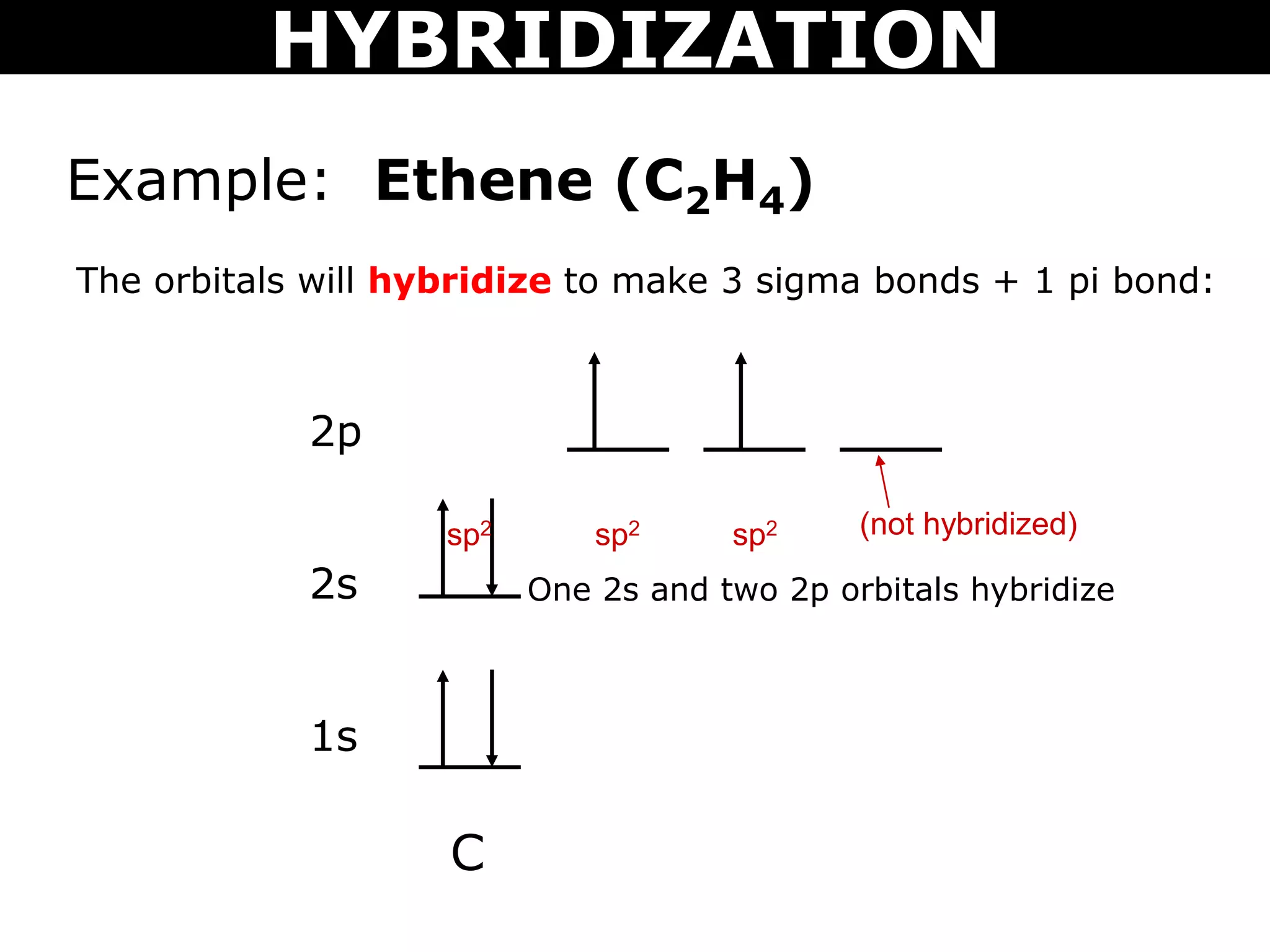
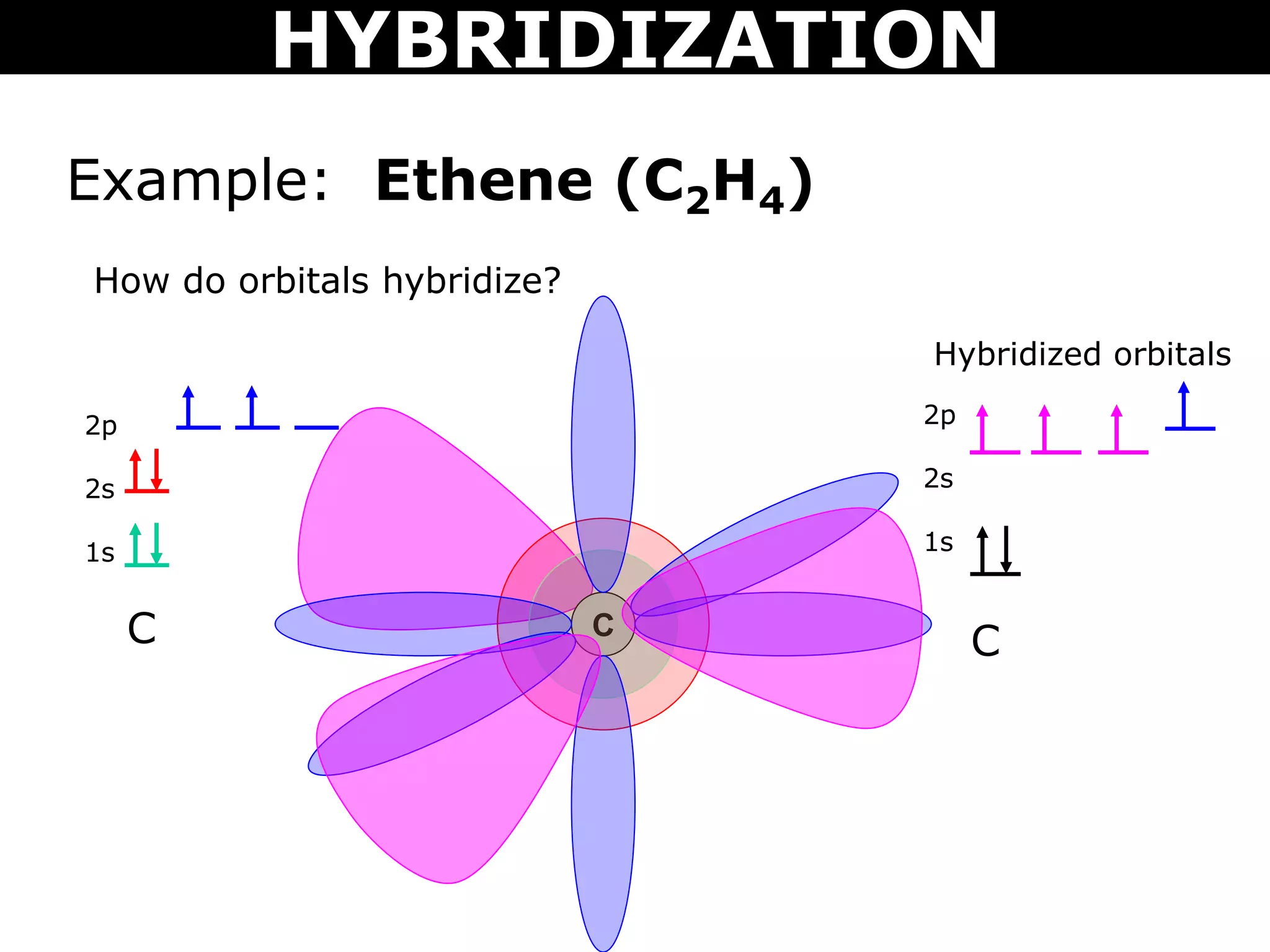
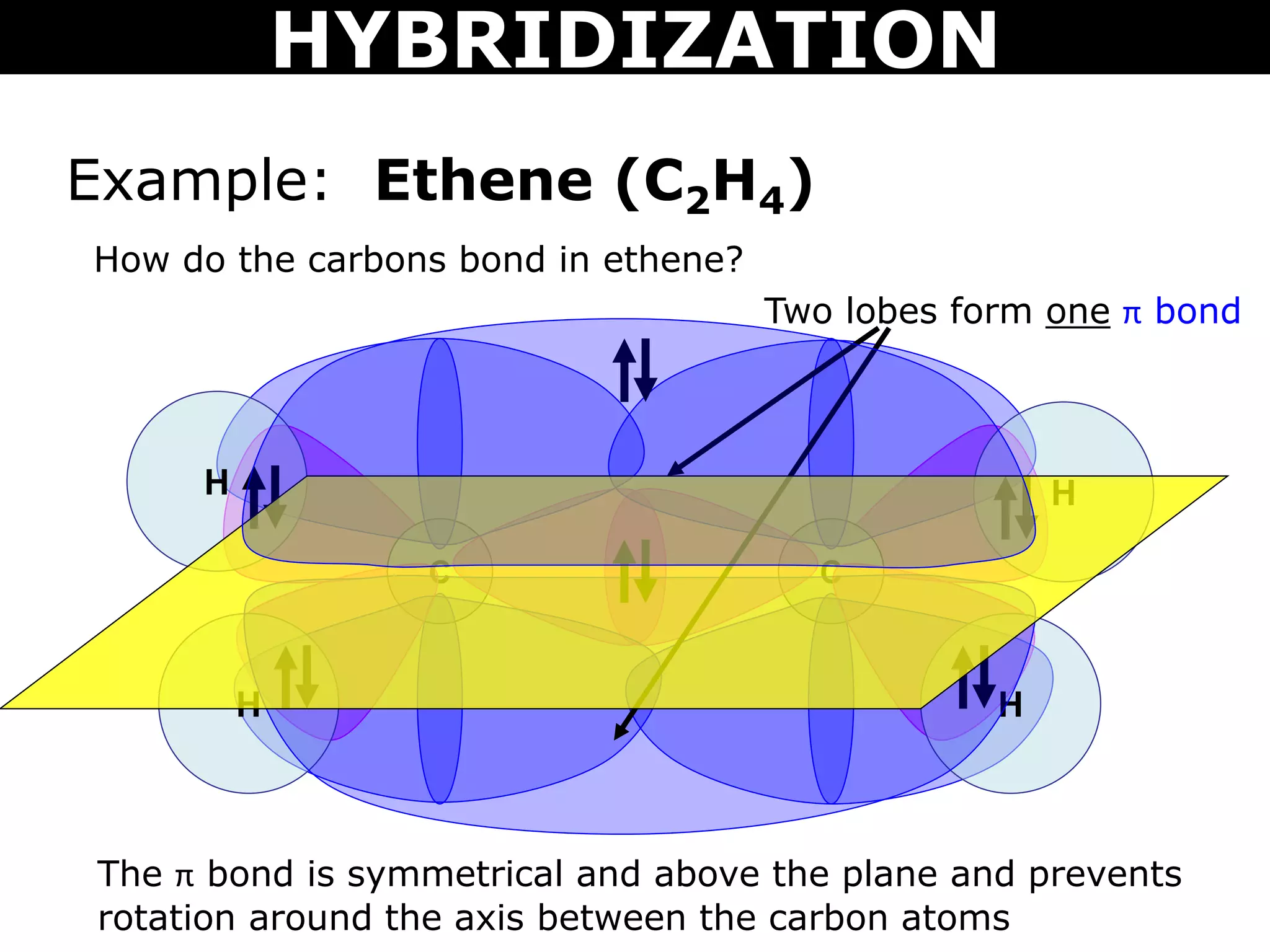
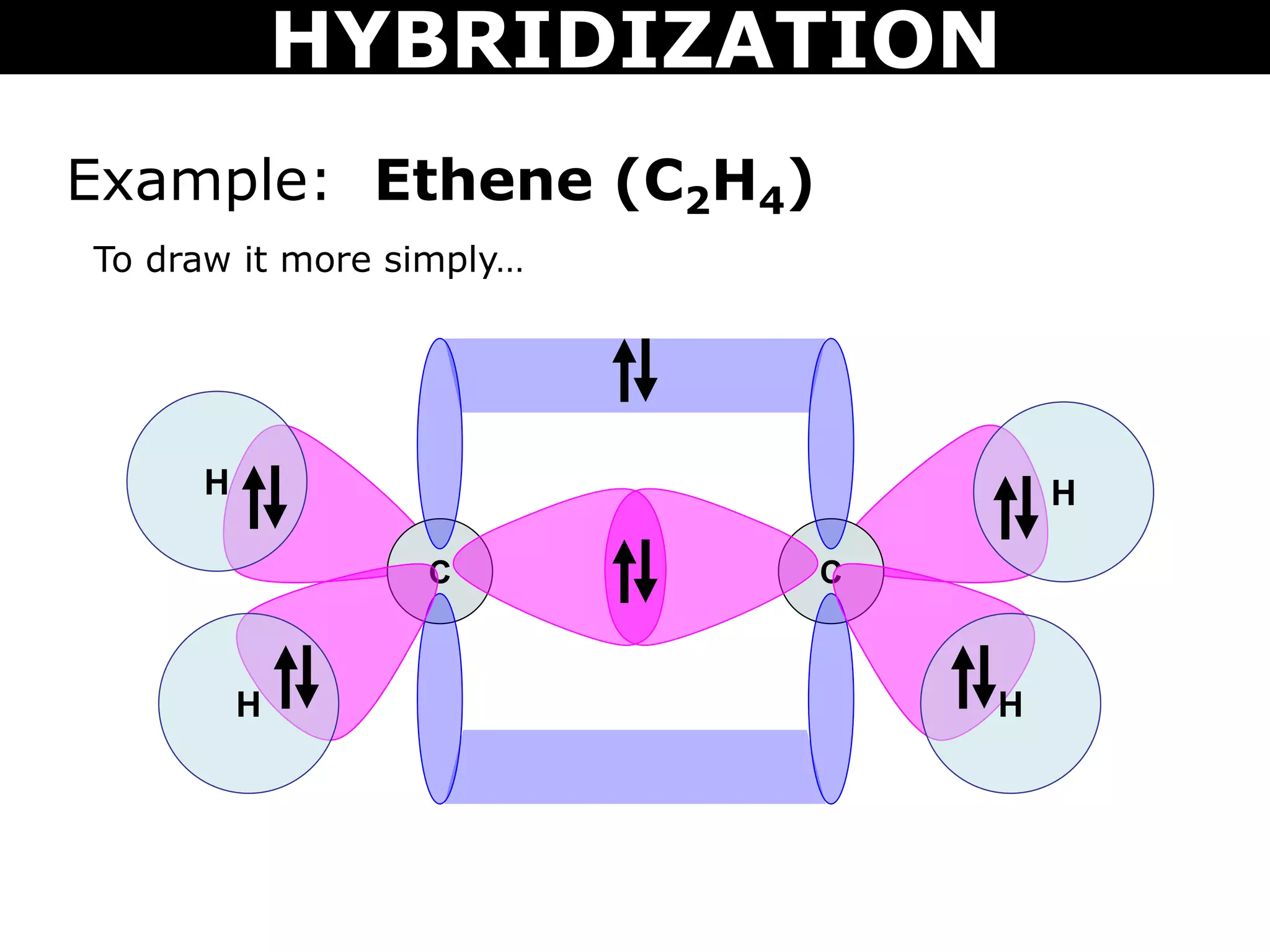
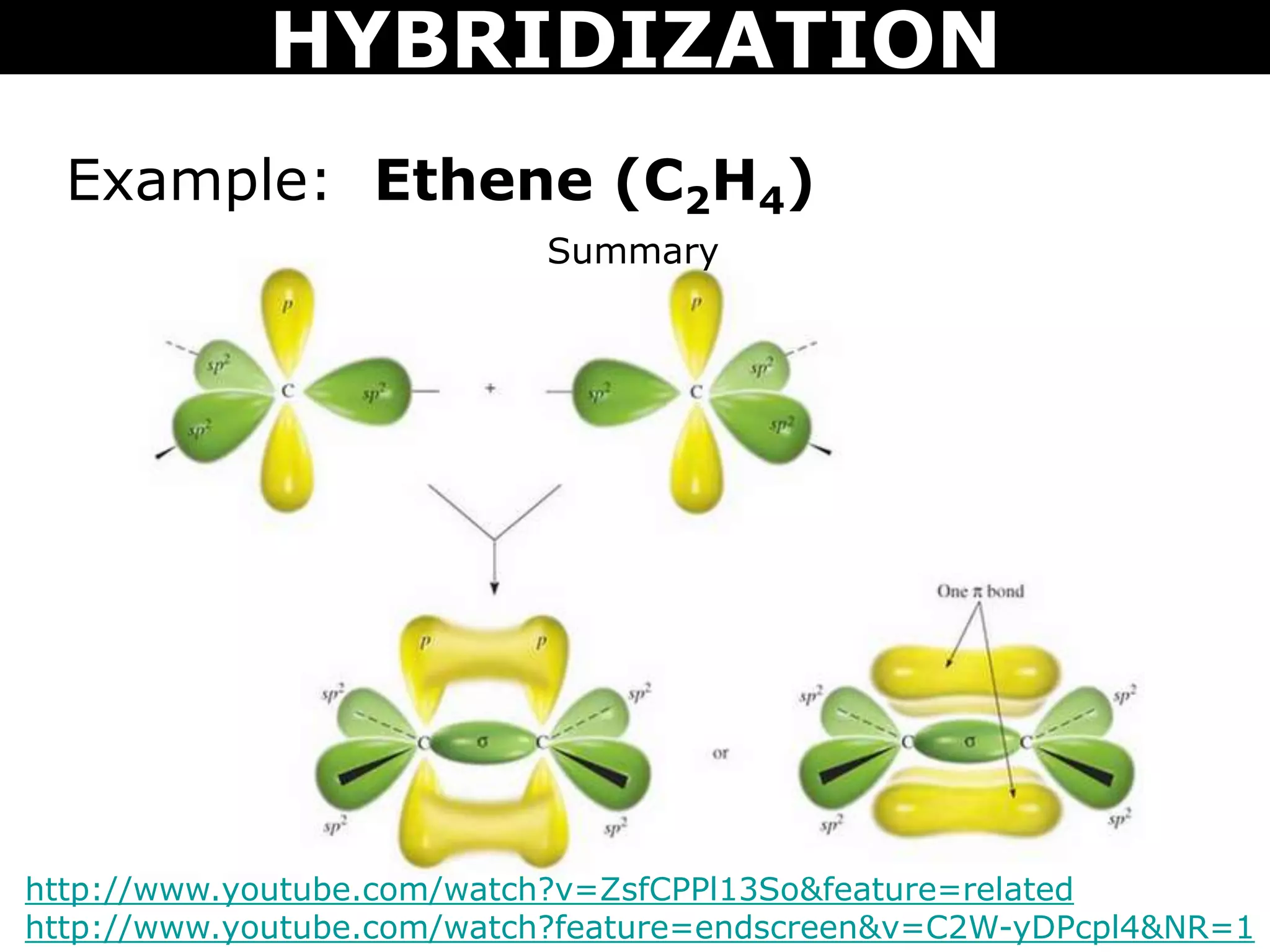
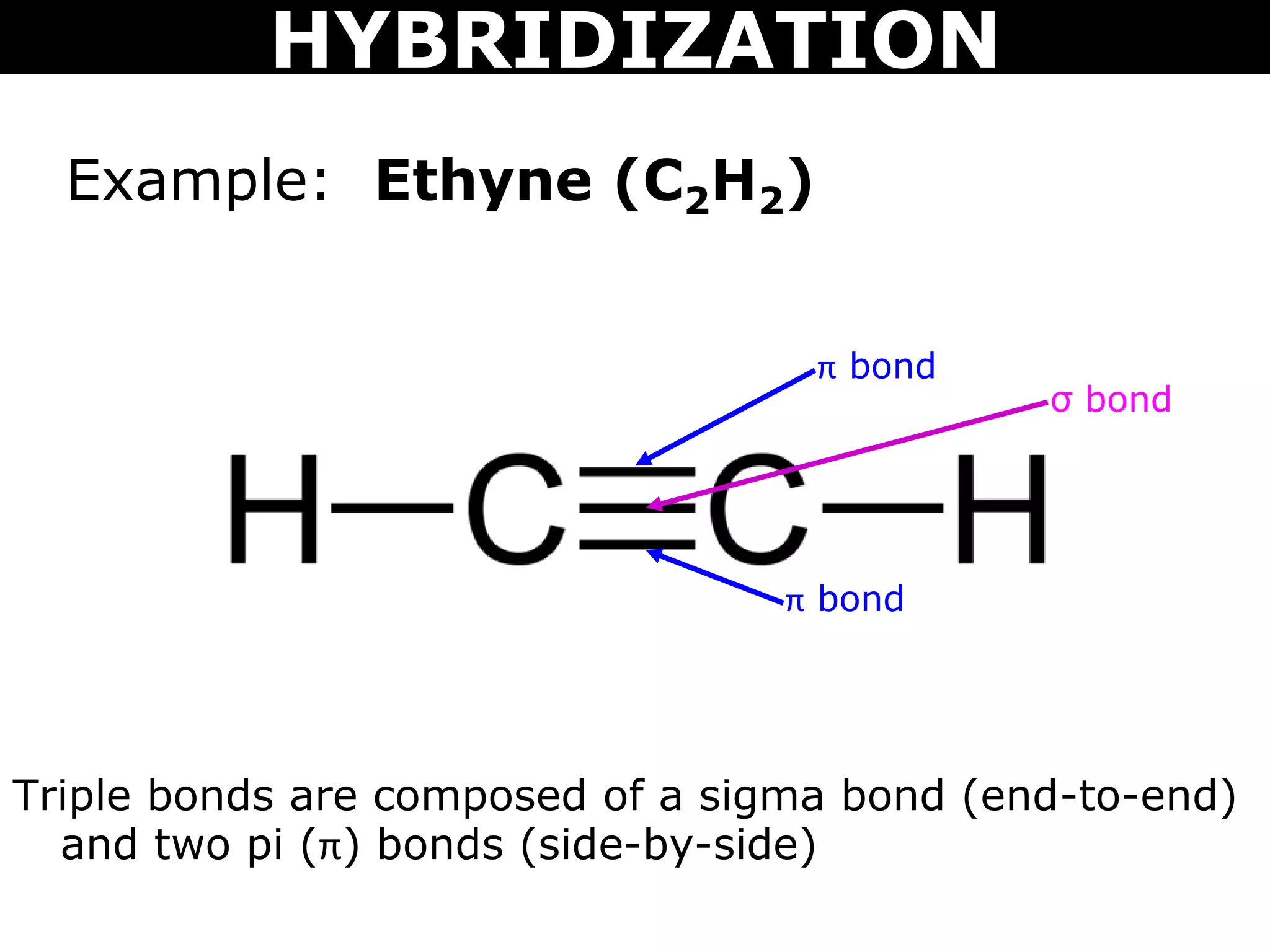
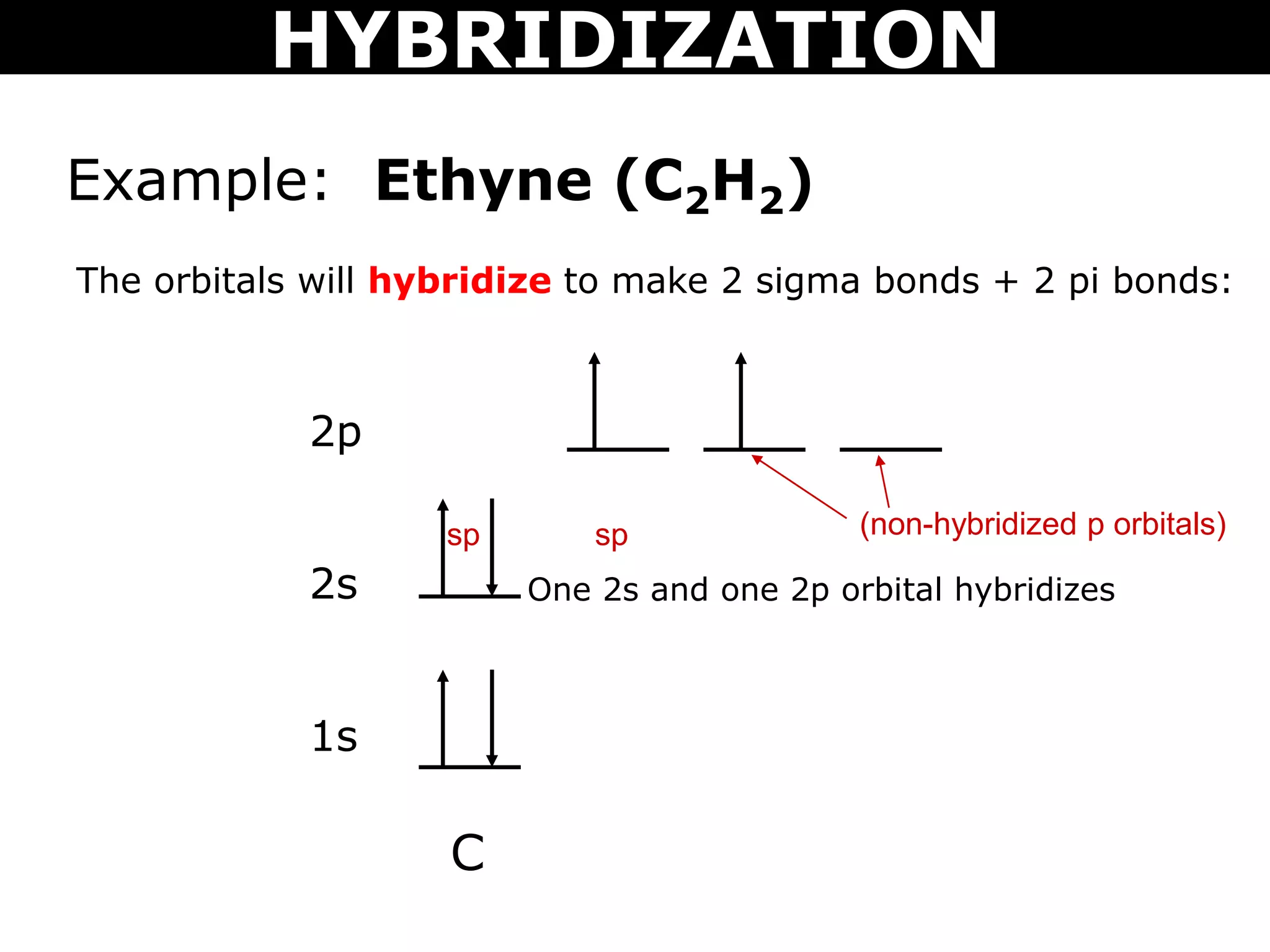
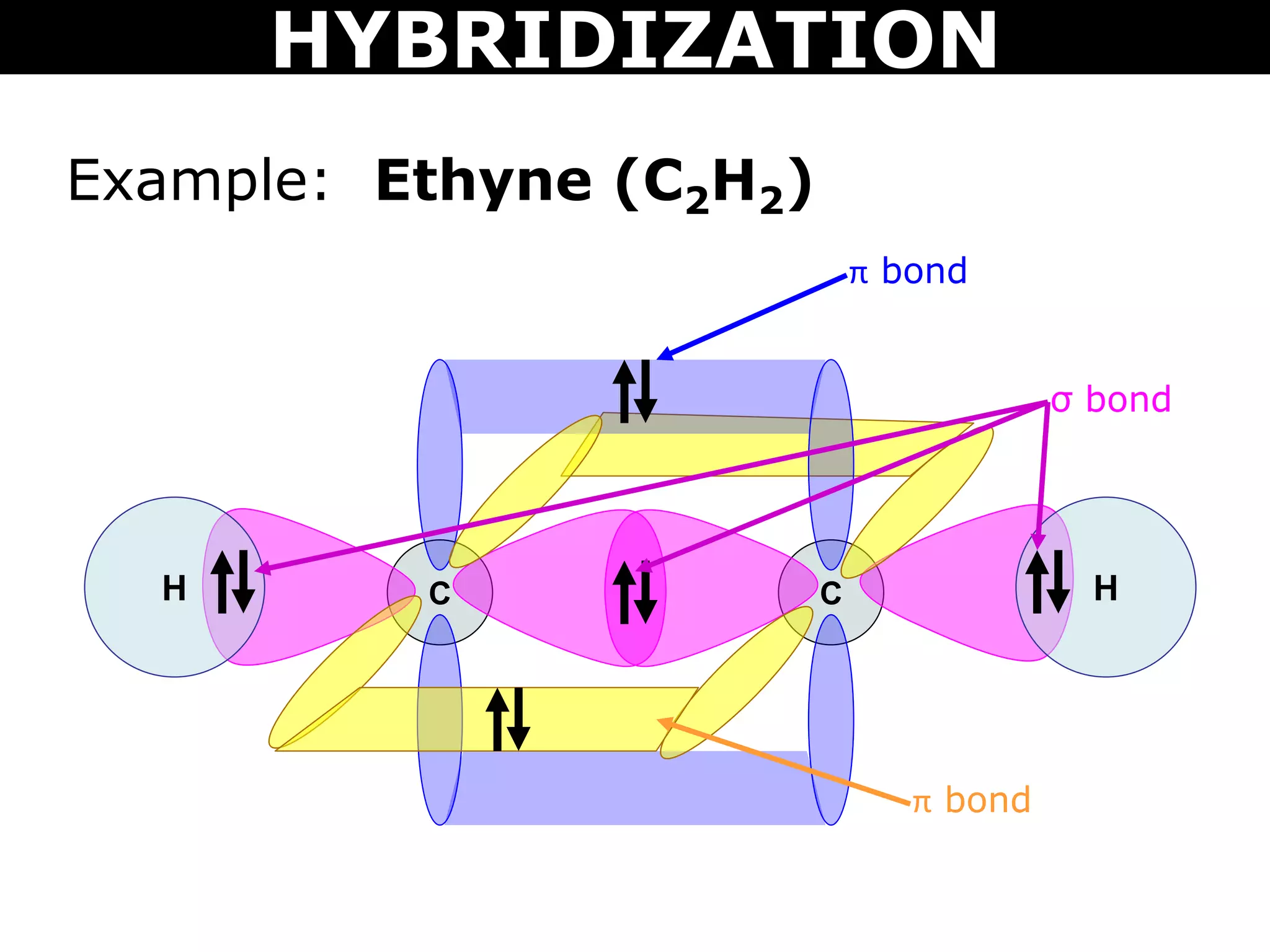
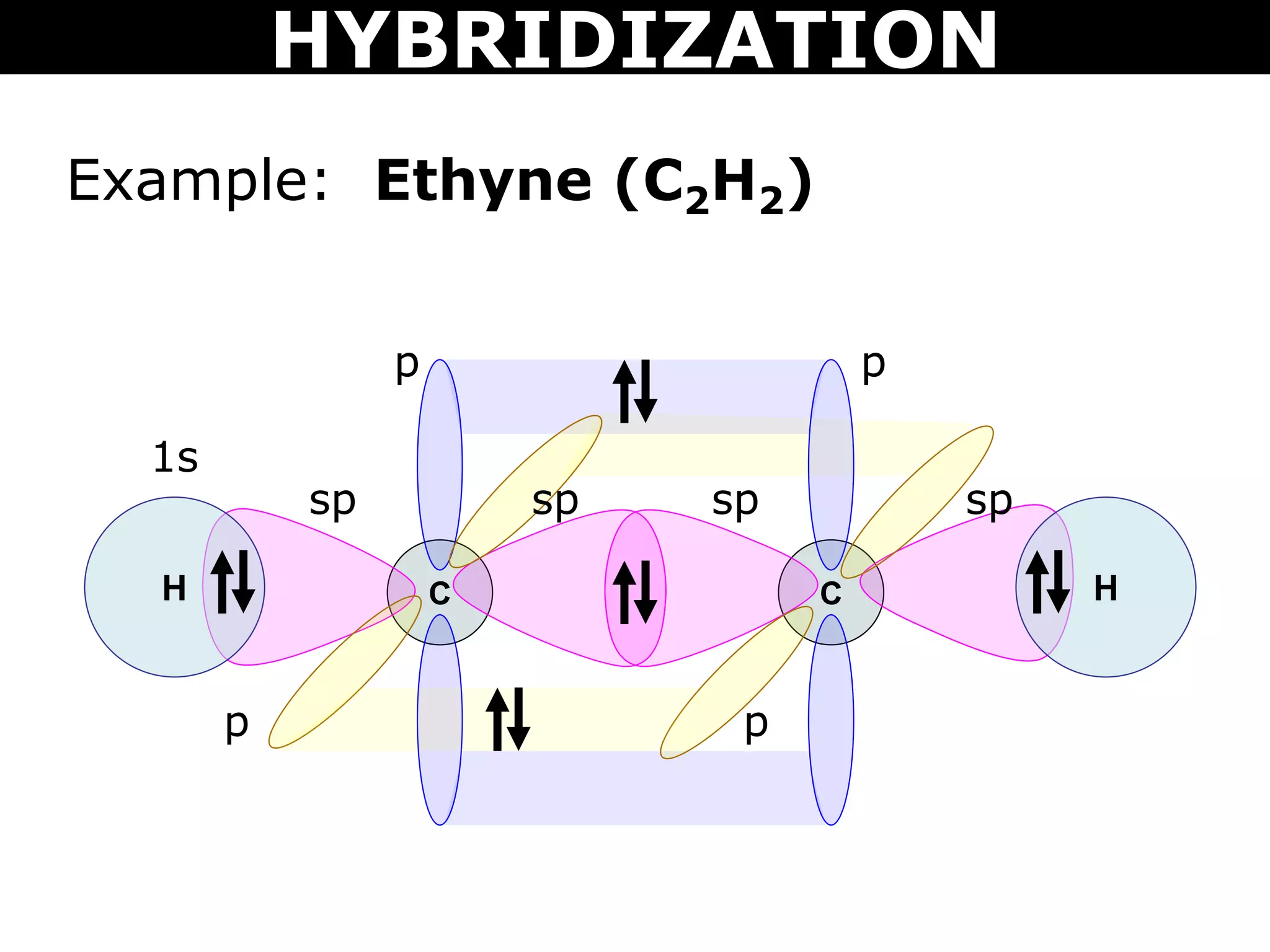
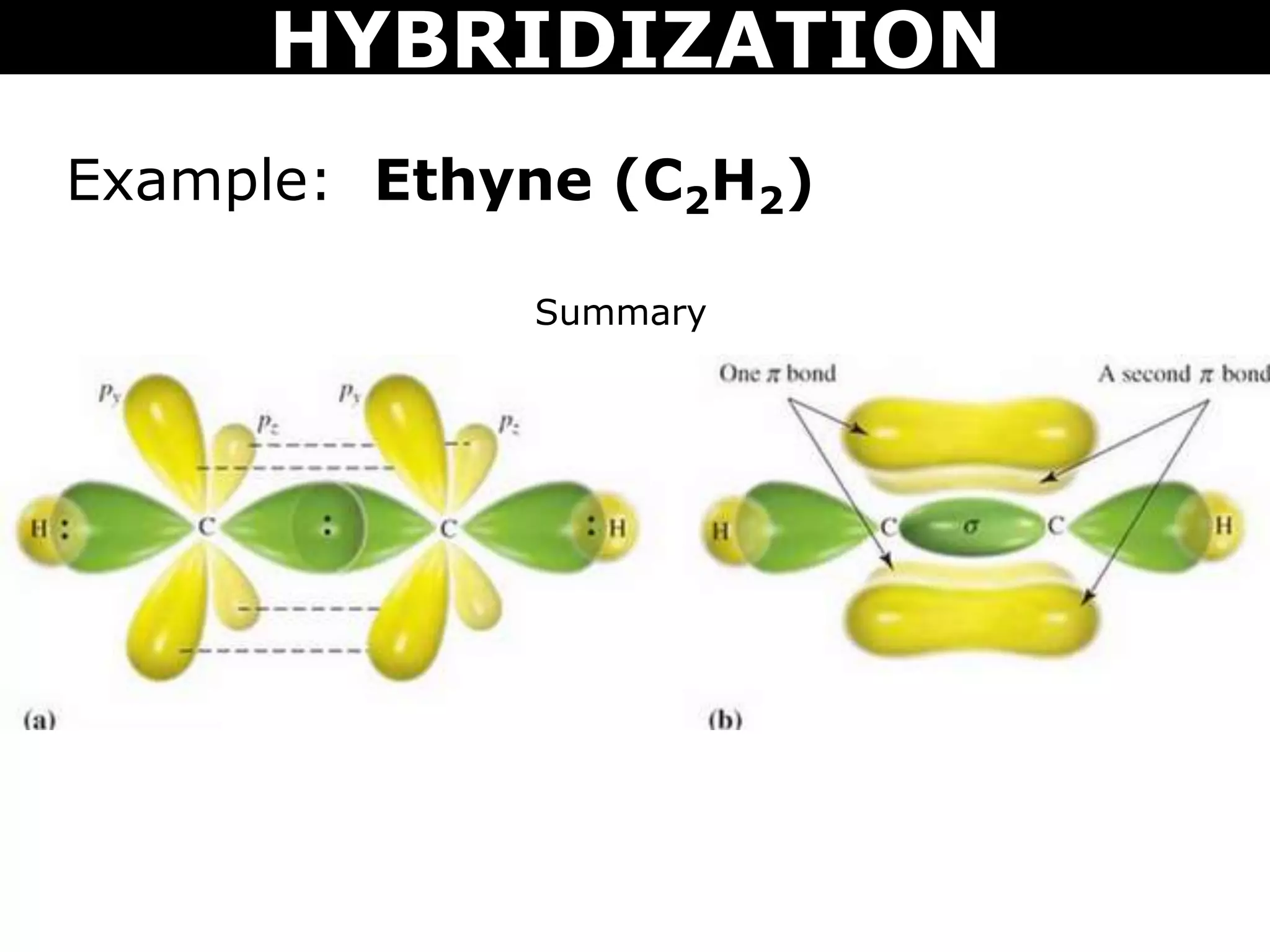
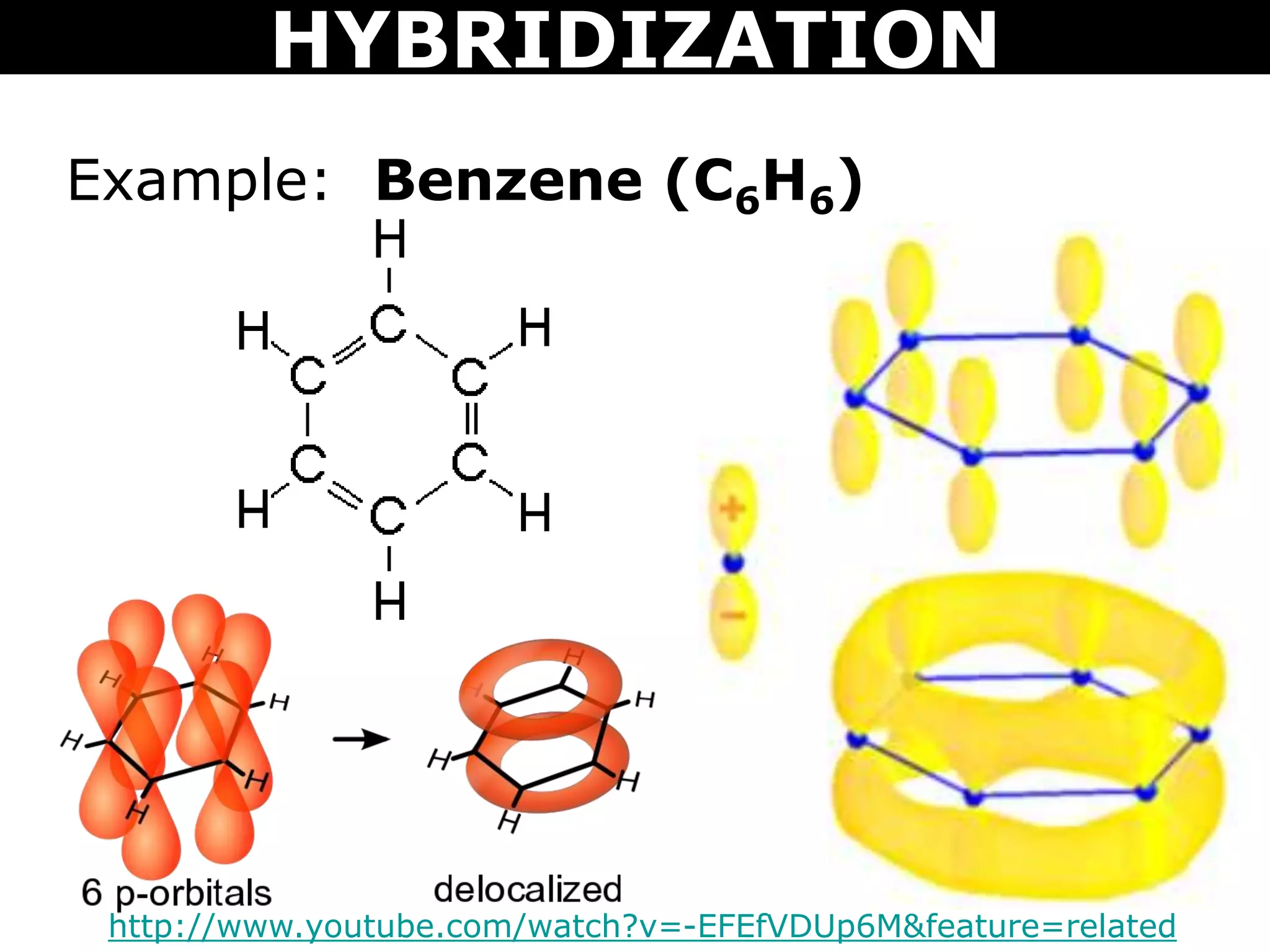
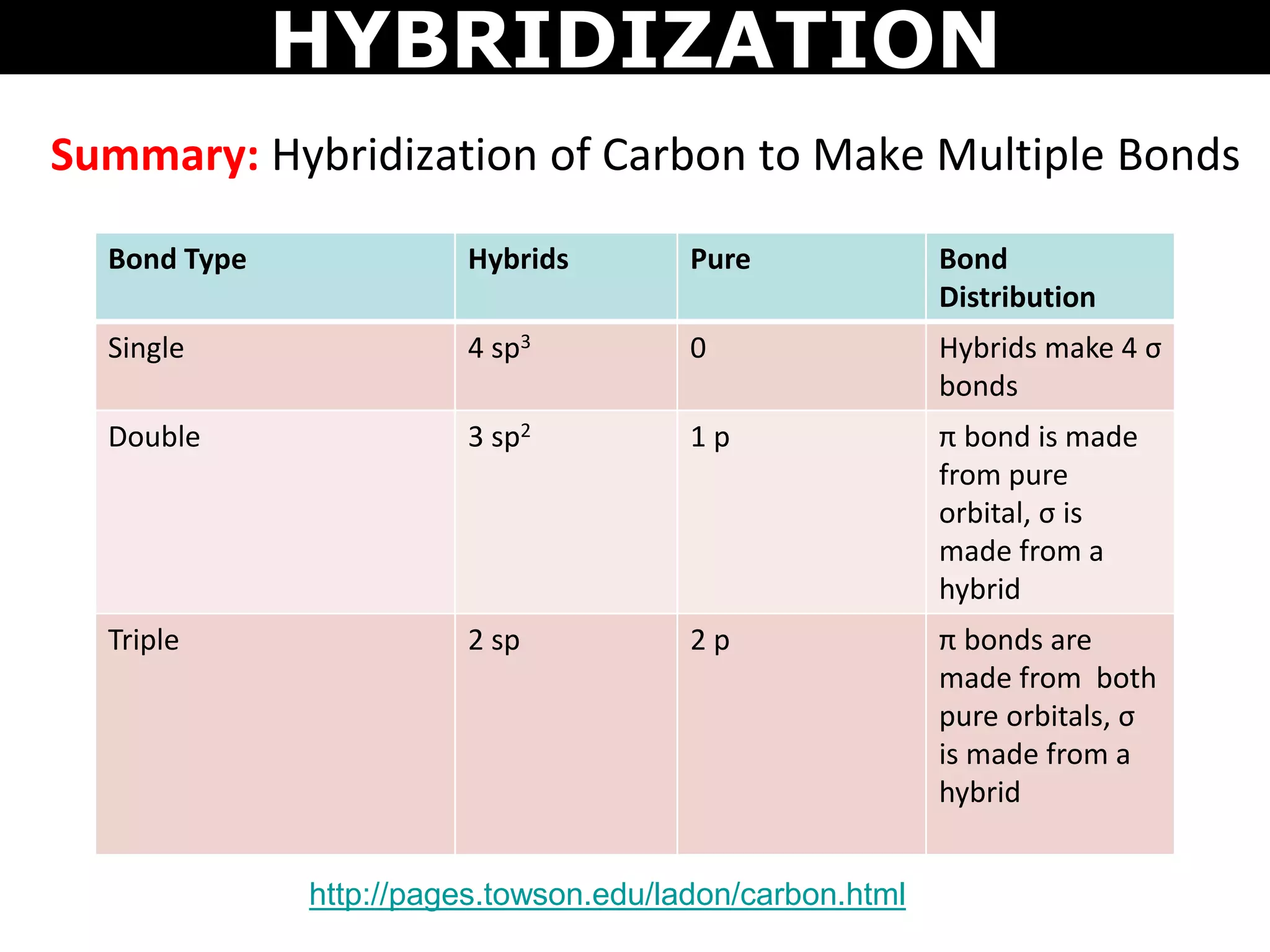
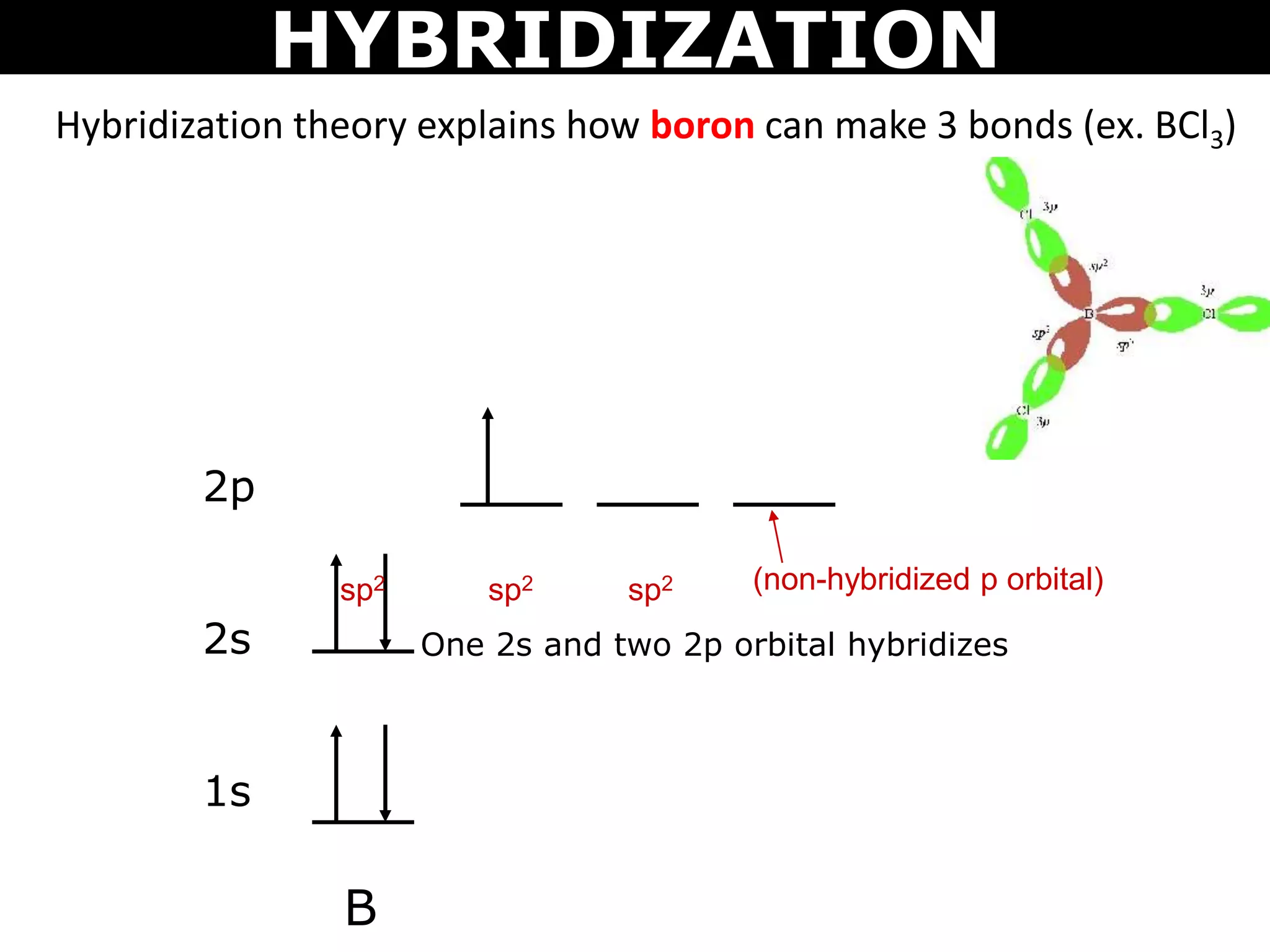
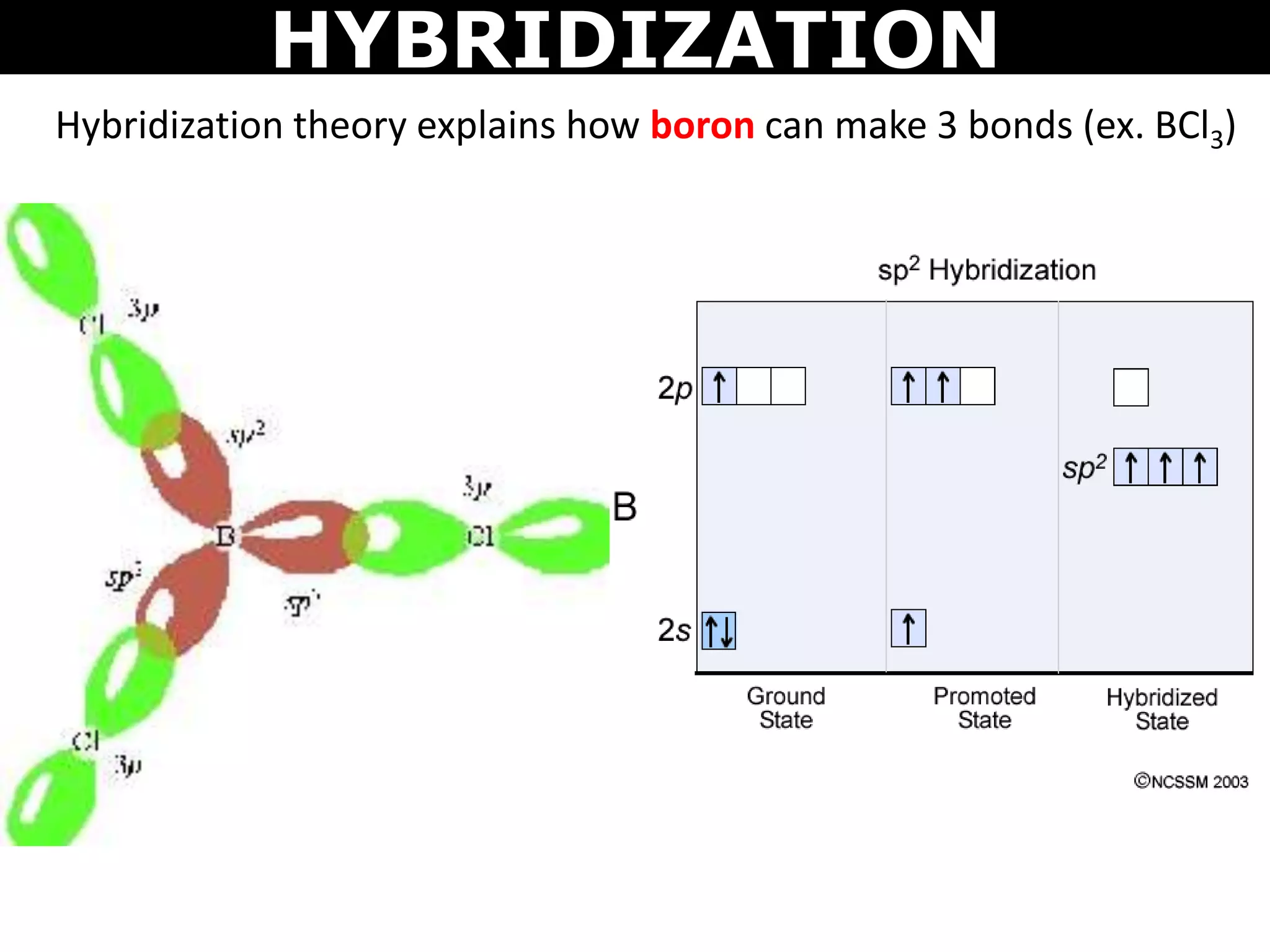
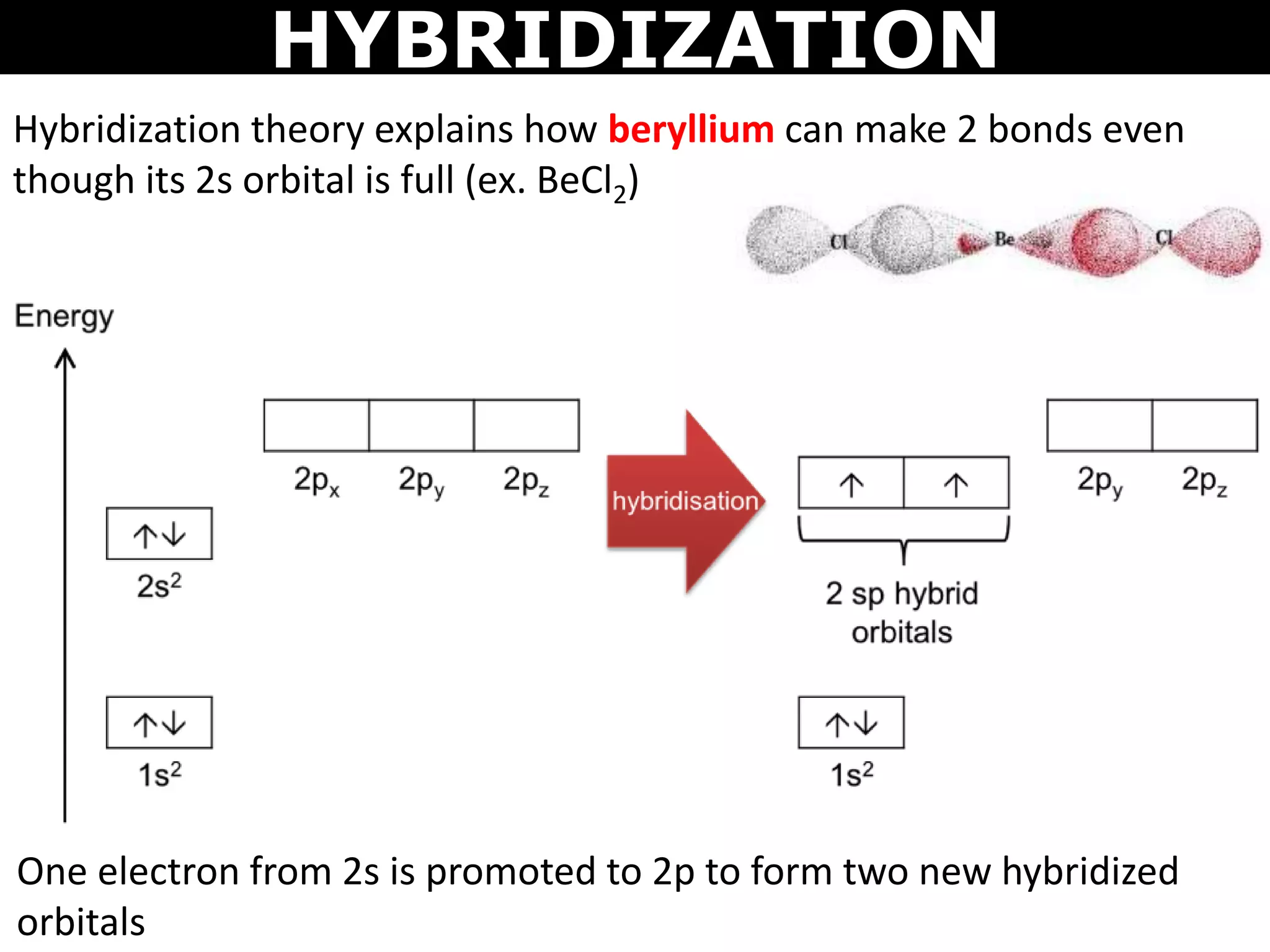
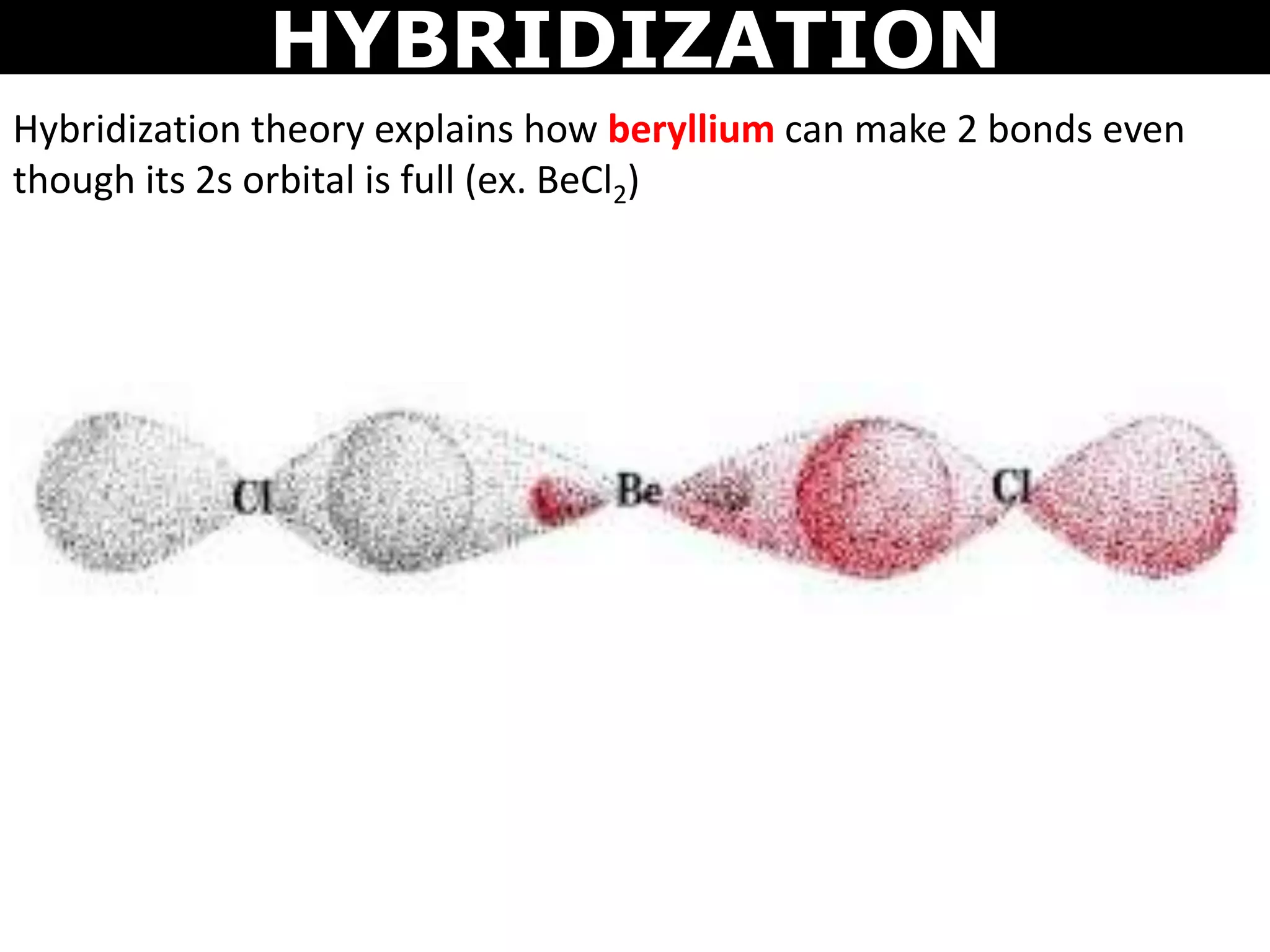
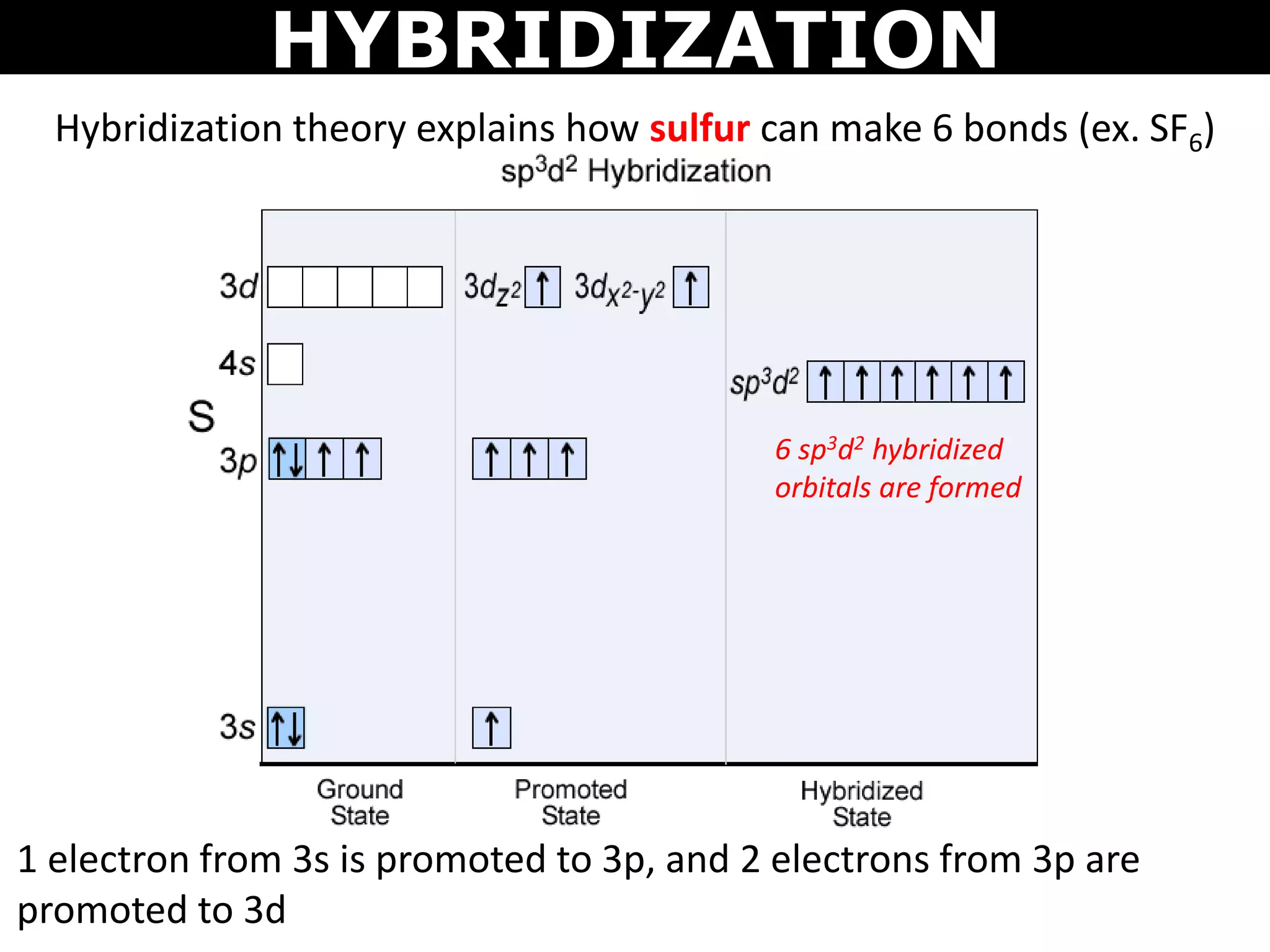
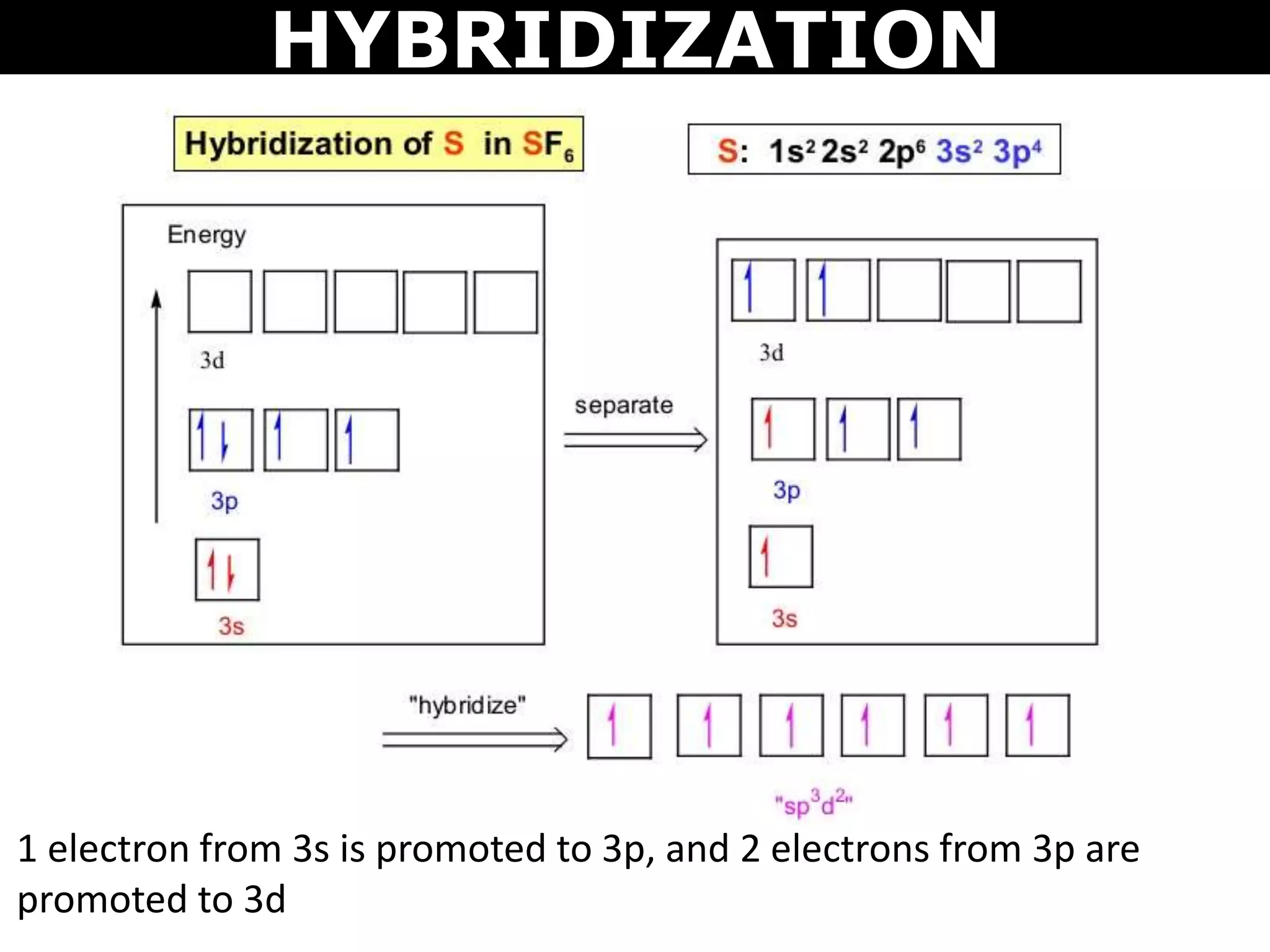
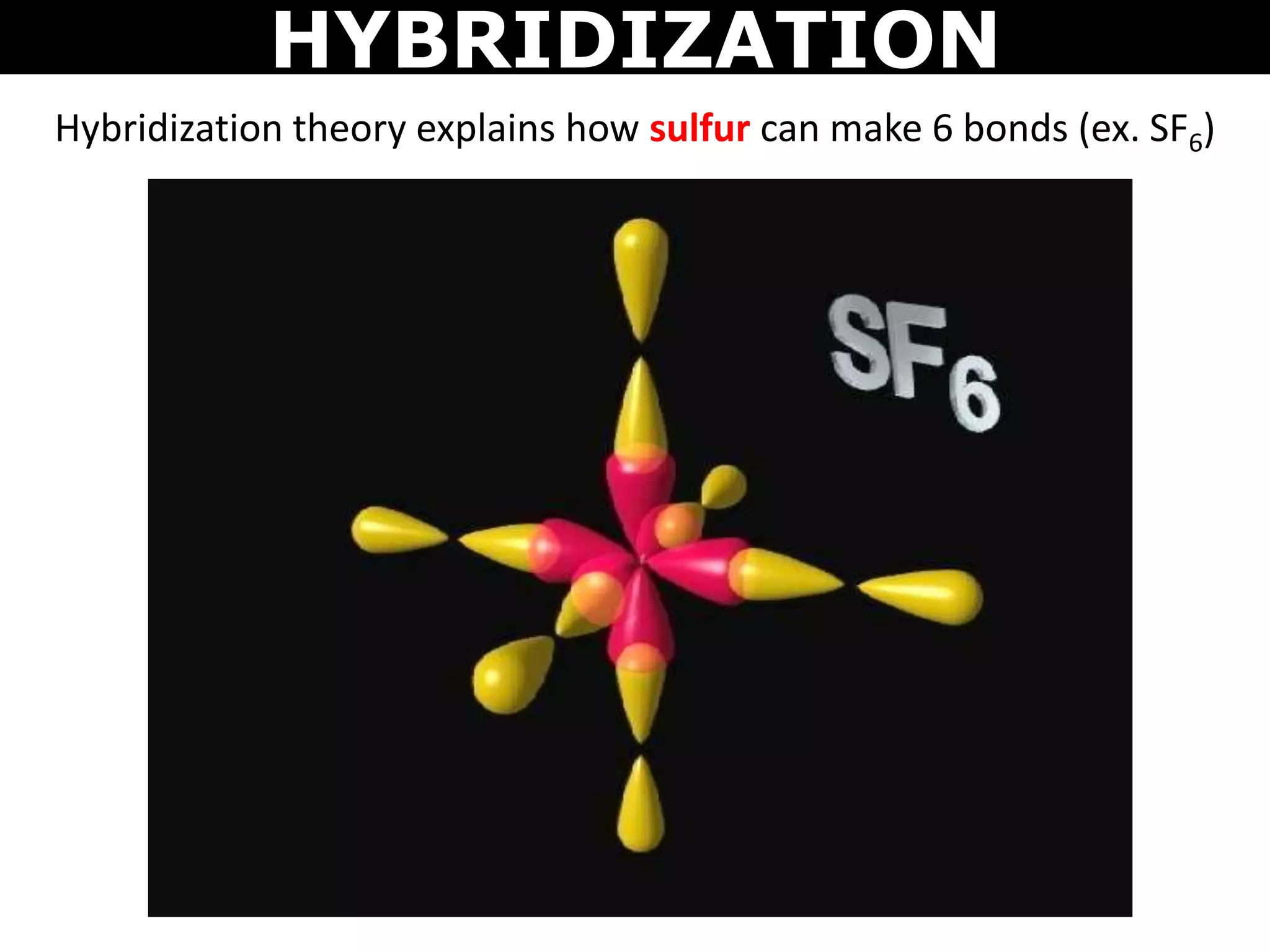
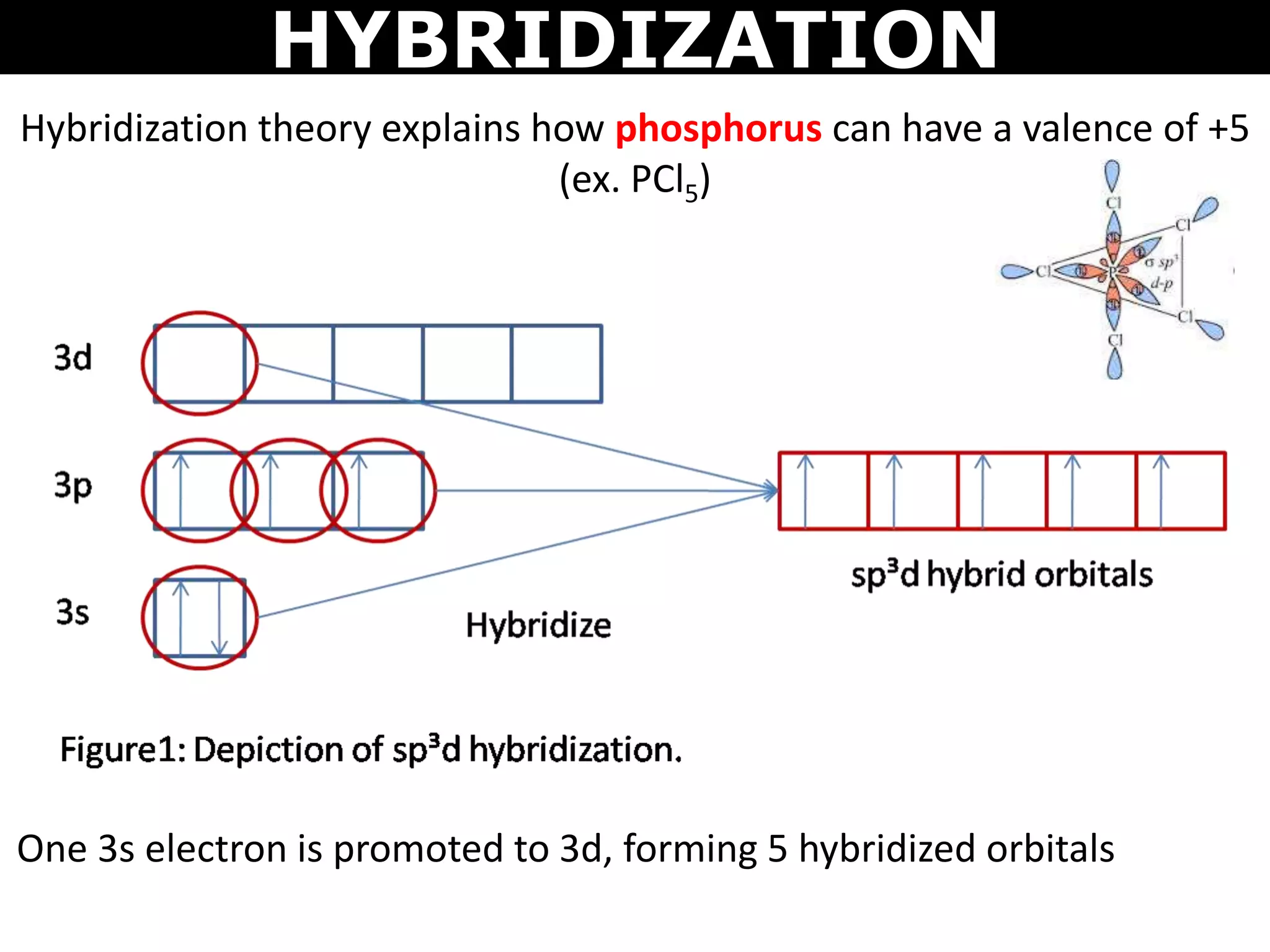
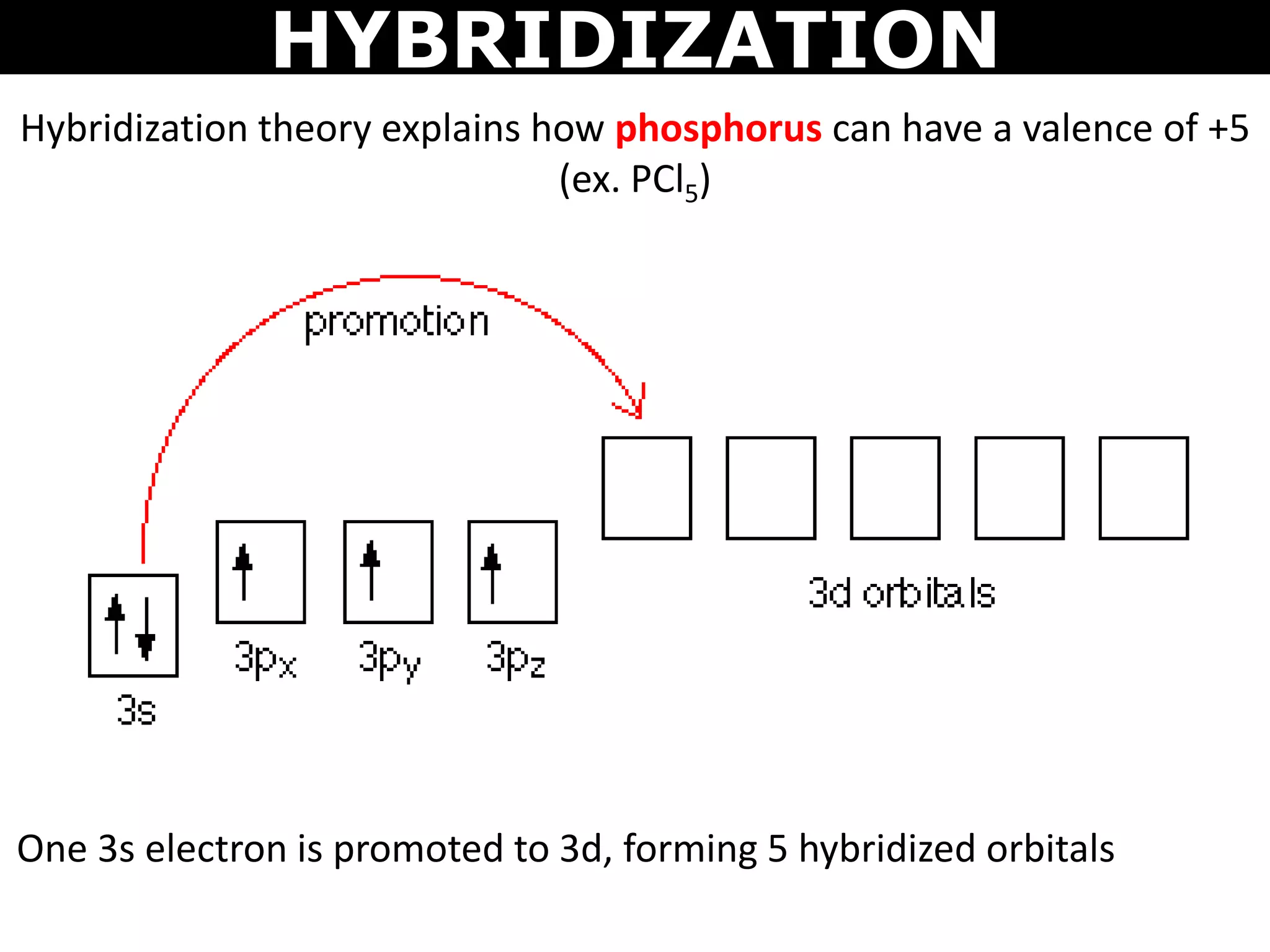
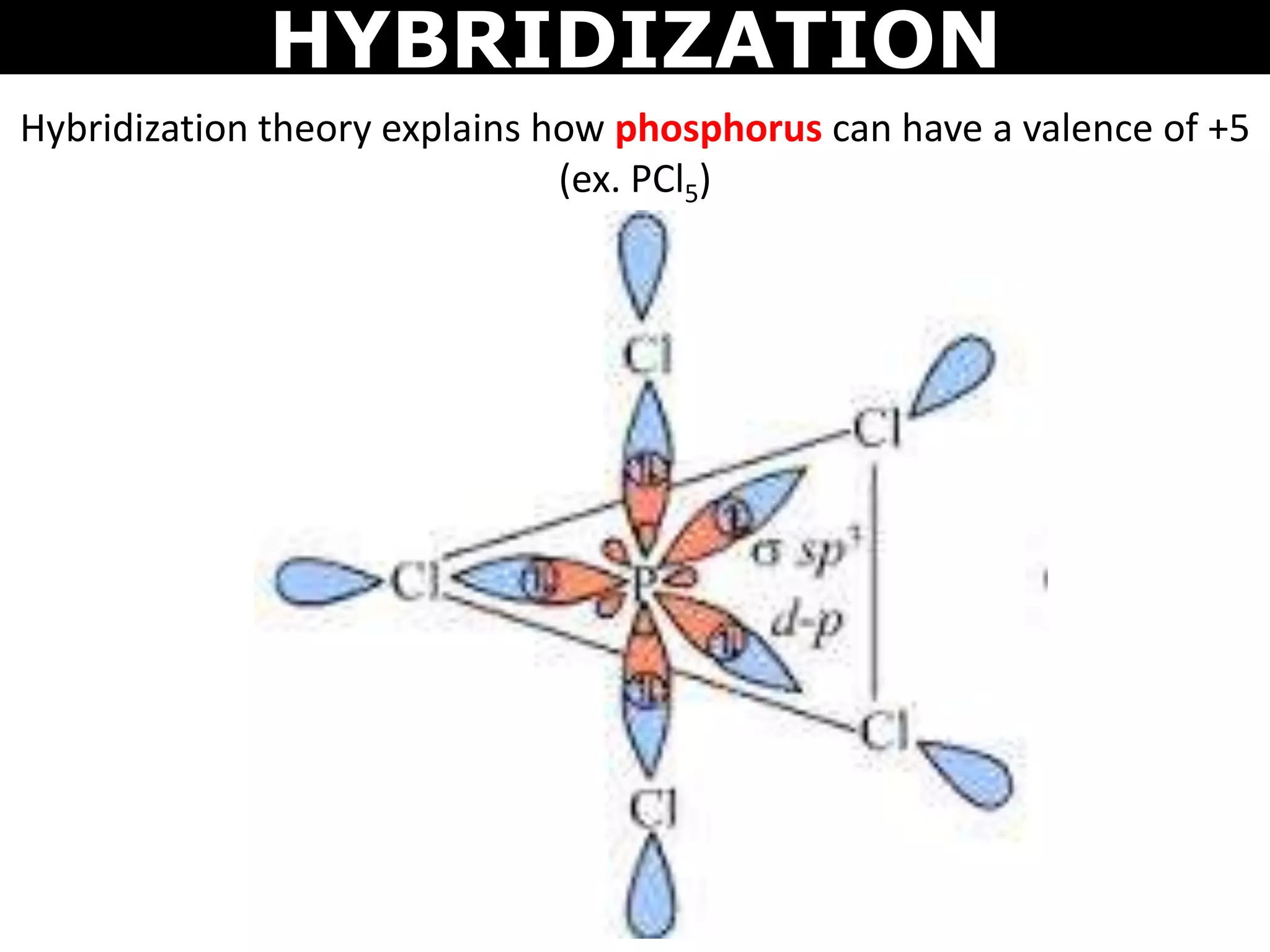
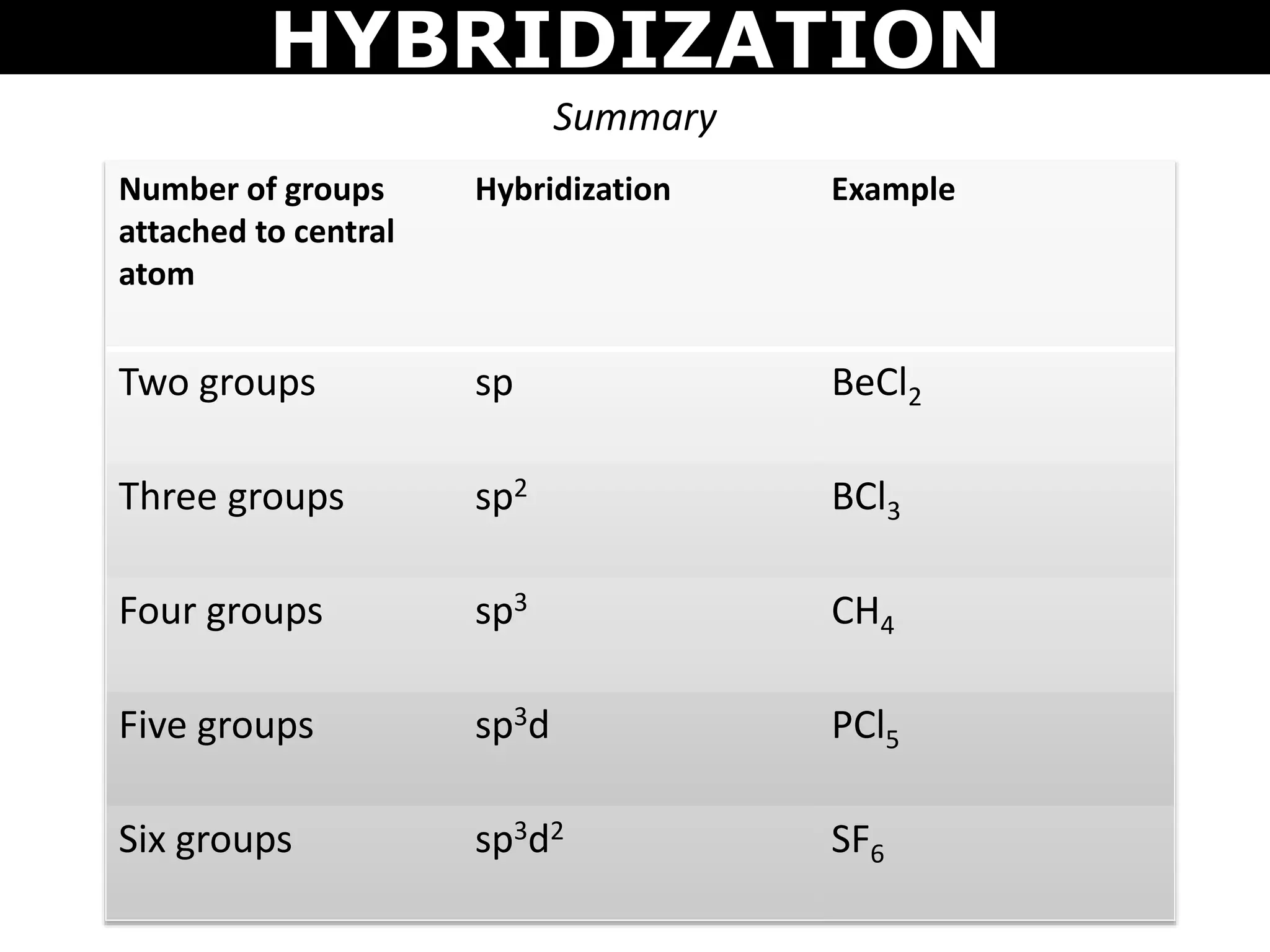
The document discusses valence bond theory and hybridization. Valence bond theory explains how covalent bonds form through the overlapping of atomic orbitals. Hybridization occurs when atomic orbitals combine to form new hybrid orbitals. This allows atoms to form more bonds than their valence shell configuration suggests. Carbon can form 4 bonds through sp3 hybridization where one 2s and three 2p orbitals combine. Hybridization also explains bonding in molecules such as methane (CH4), ethene (C2H4), ethyne (C2H2), and benzene (C6H6). It further discusses how hybridization allows atoms like boron, beryllium, sulfur, and phosphorus to attain unusual bonding configurations