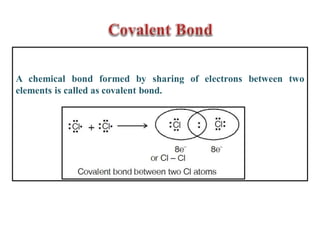
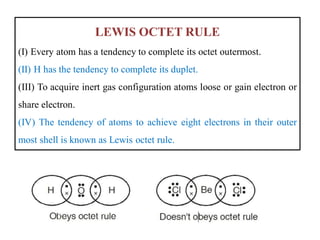
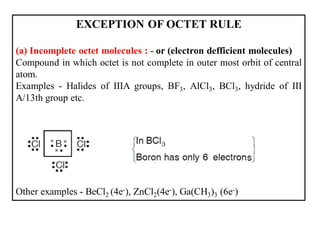
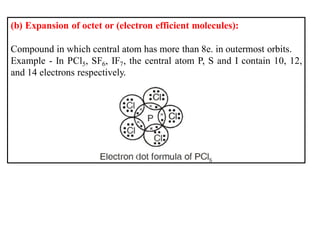
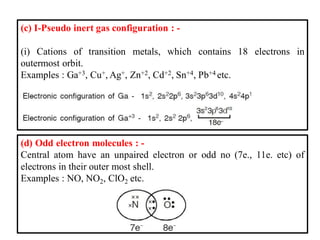
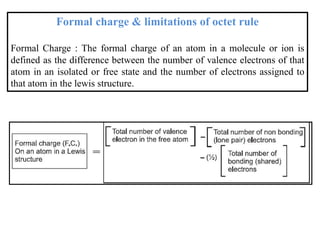
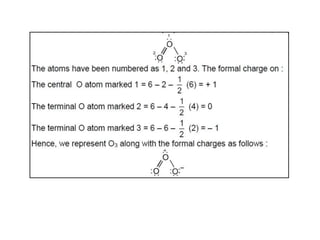
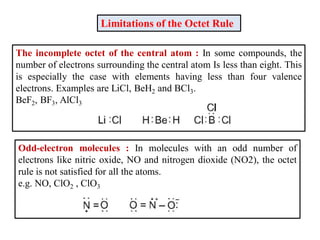
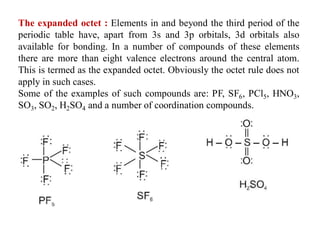
This document discusses the octet rule for chemical bonding. It states that the octet rule describes the tendency of atoms to attain noble gas configurations by gaining, losing or sharing electrons to acquire eight electrons in their outer shell. There are exceptions when the octet is not complete, such as with boron trifluoride (BF3), or when it is expanded as in phosphorus pentachloride (PCl5) which has 10 electrons around the phosphorus atom. The limitations of the octet rule are also outlined.