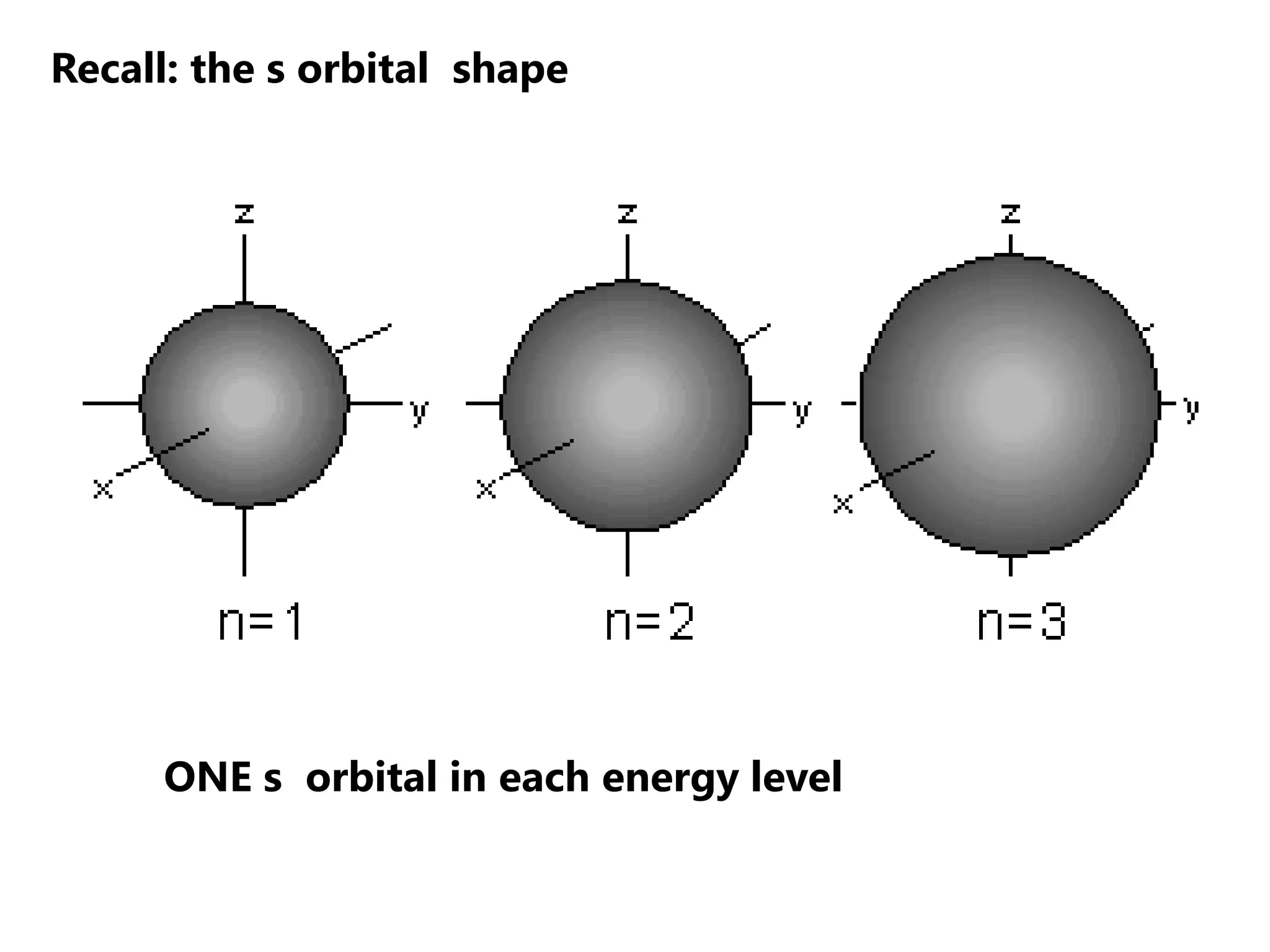
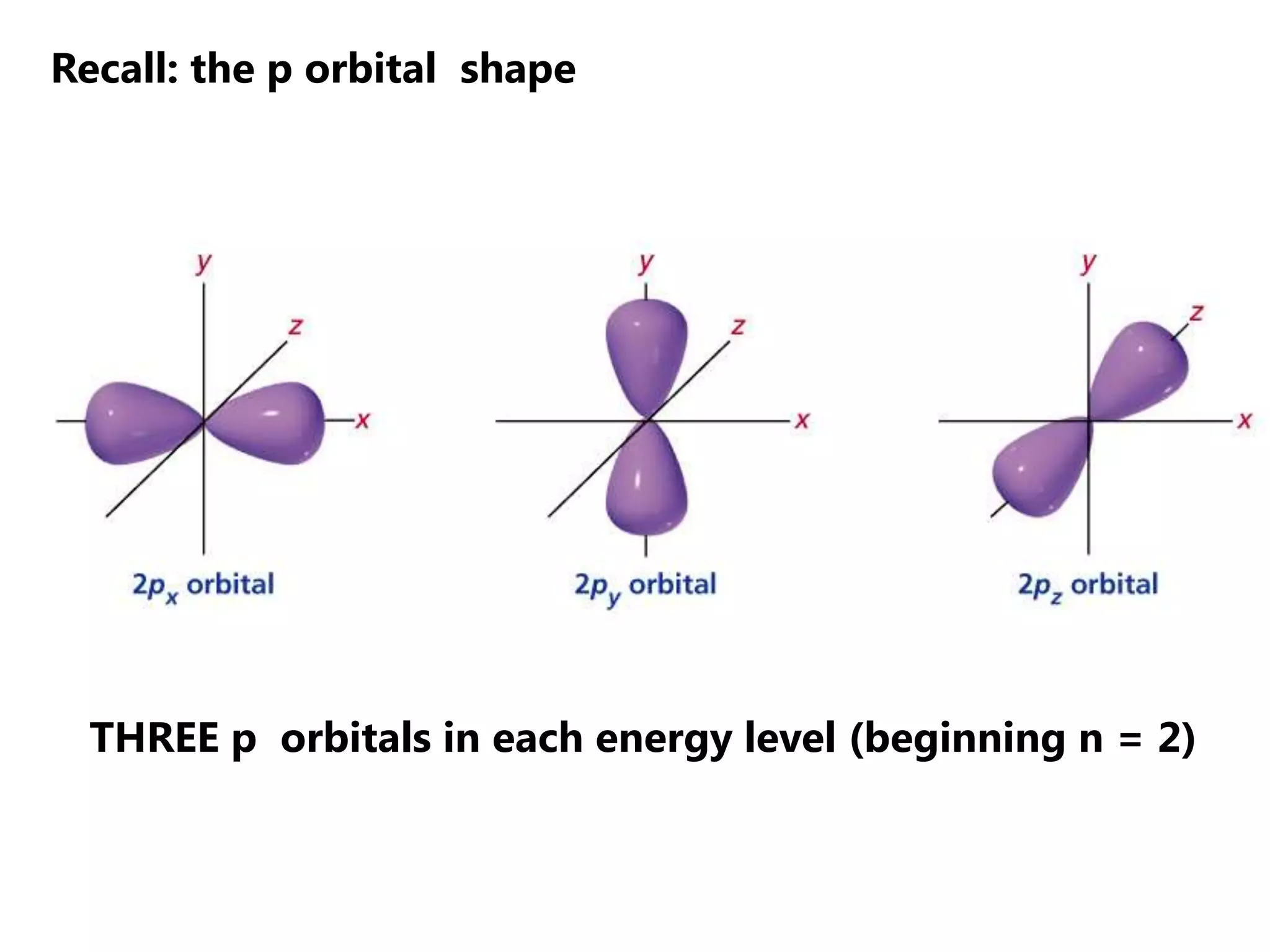
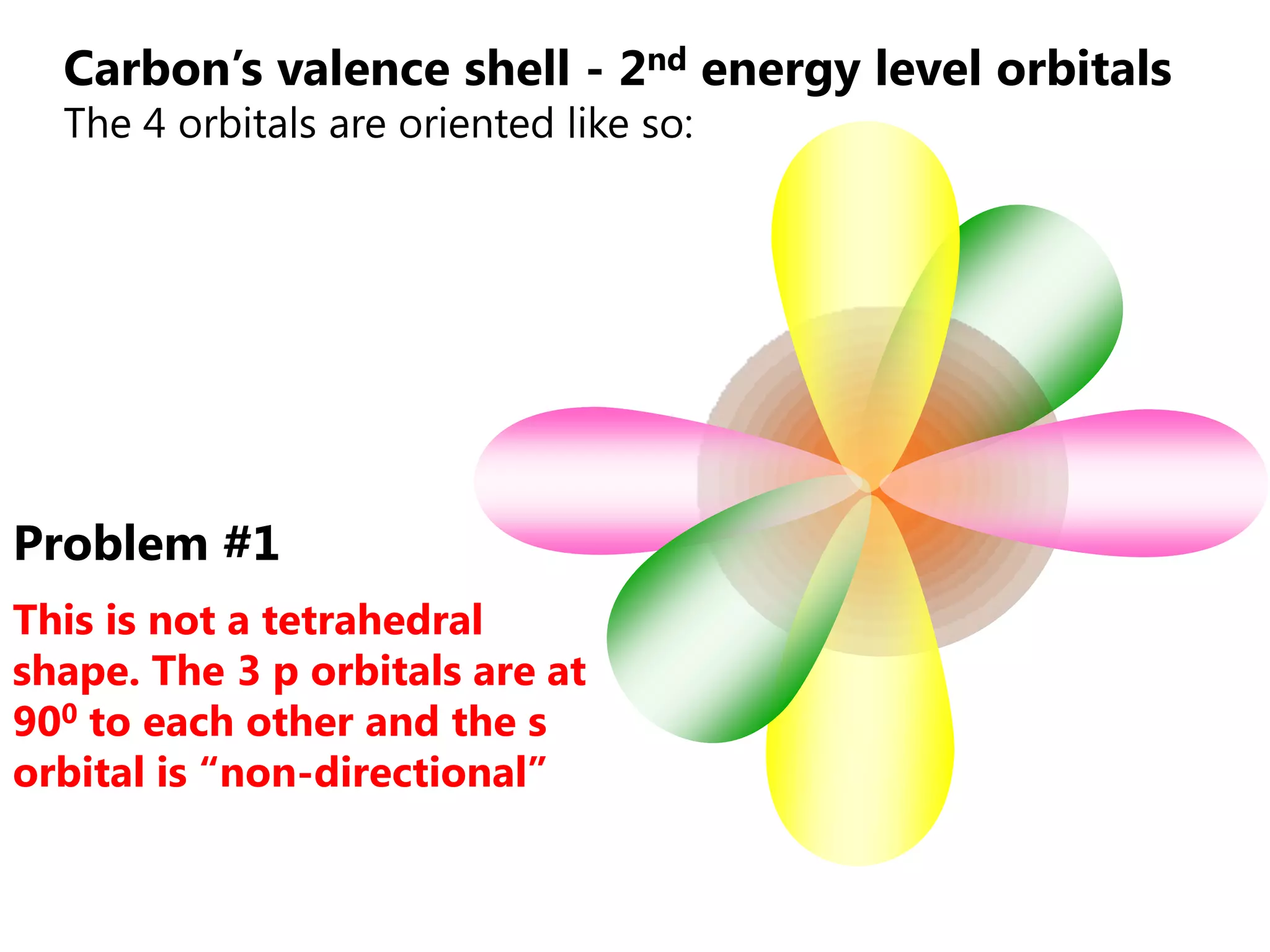
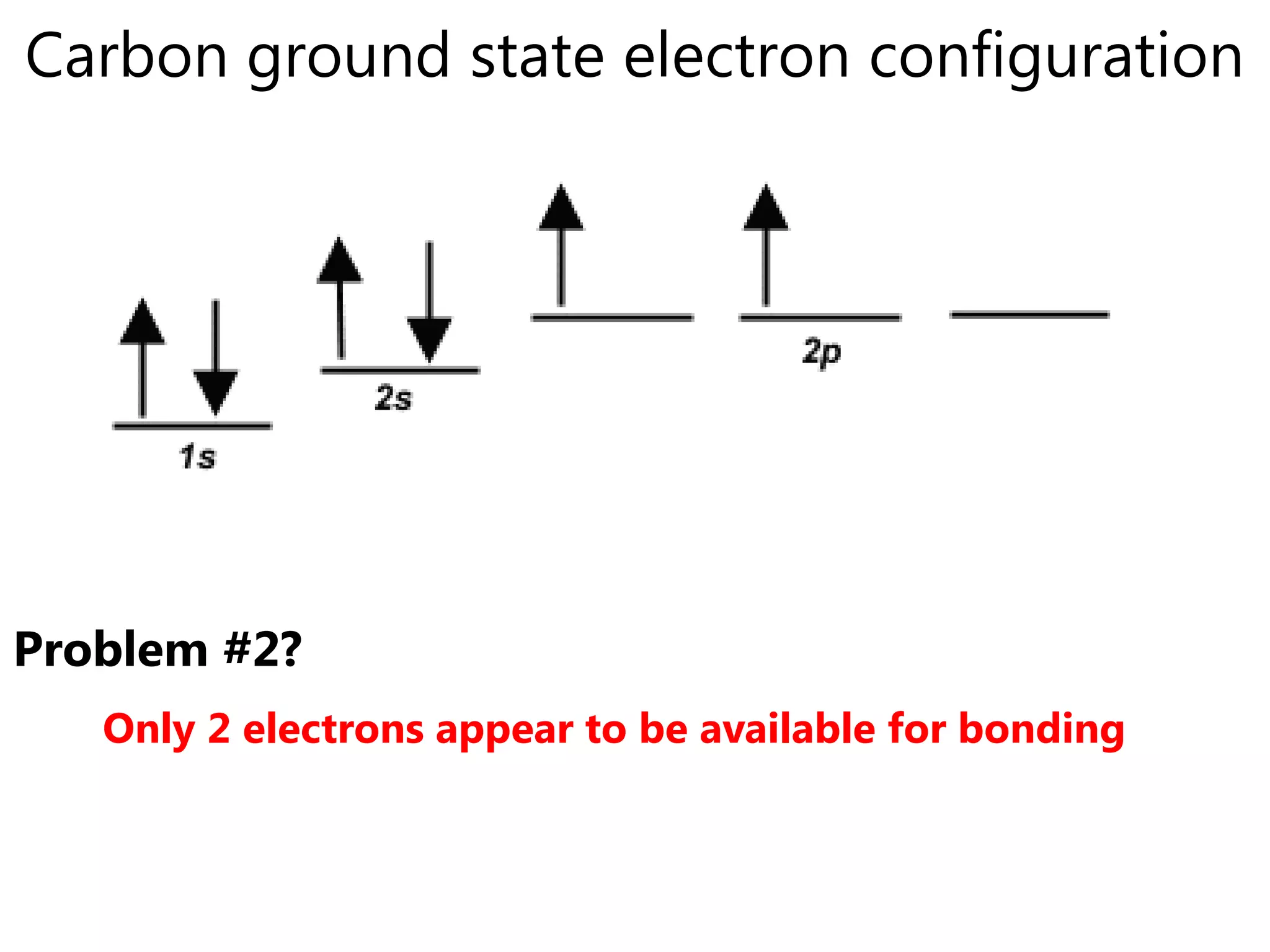
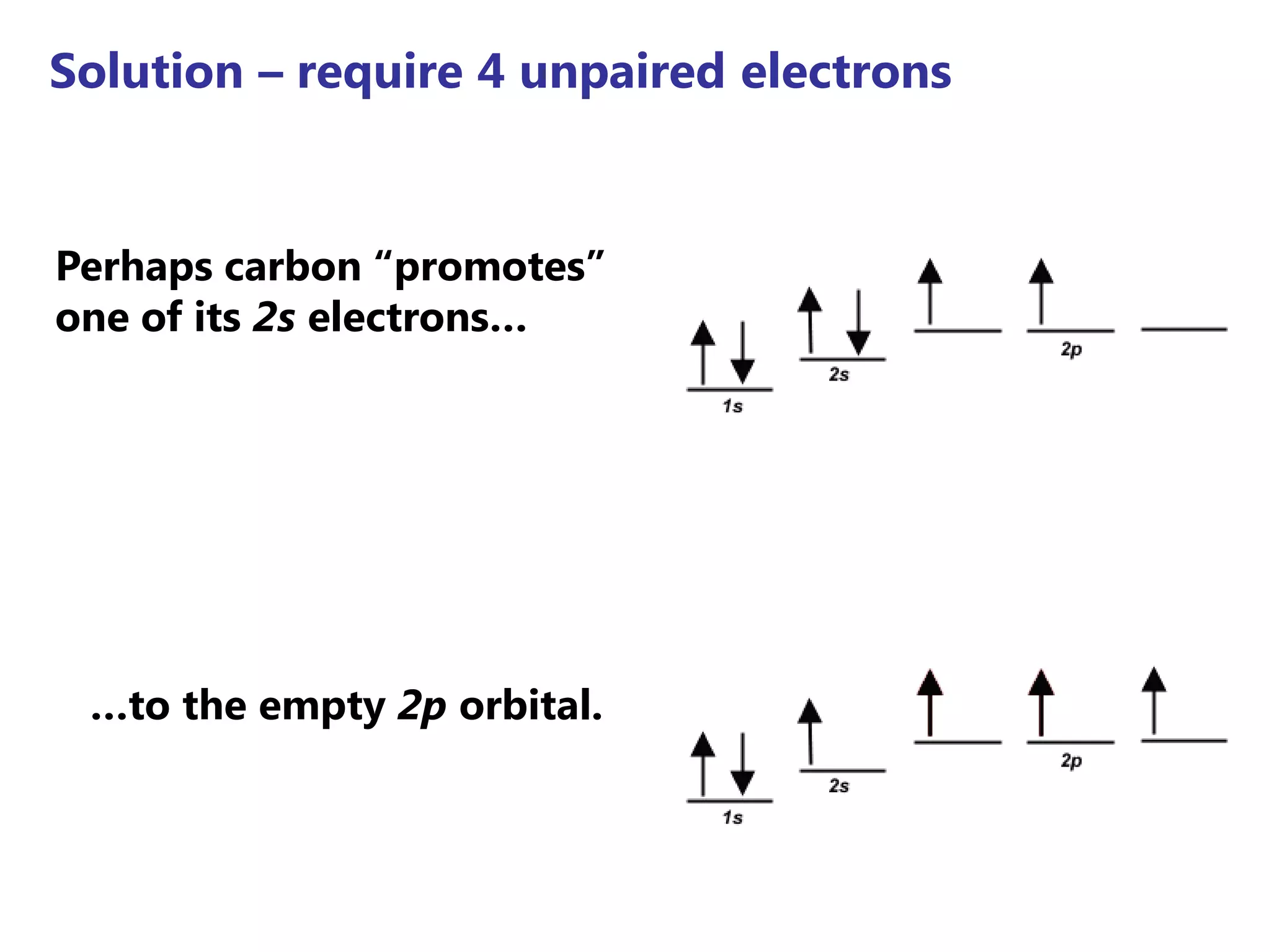
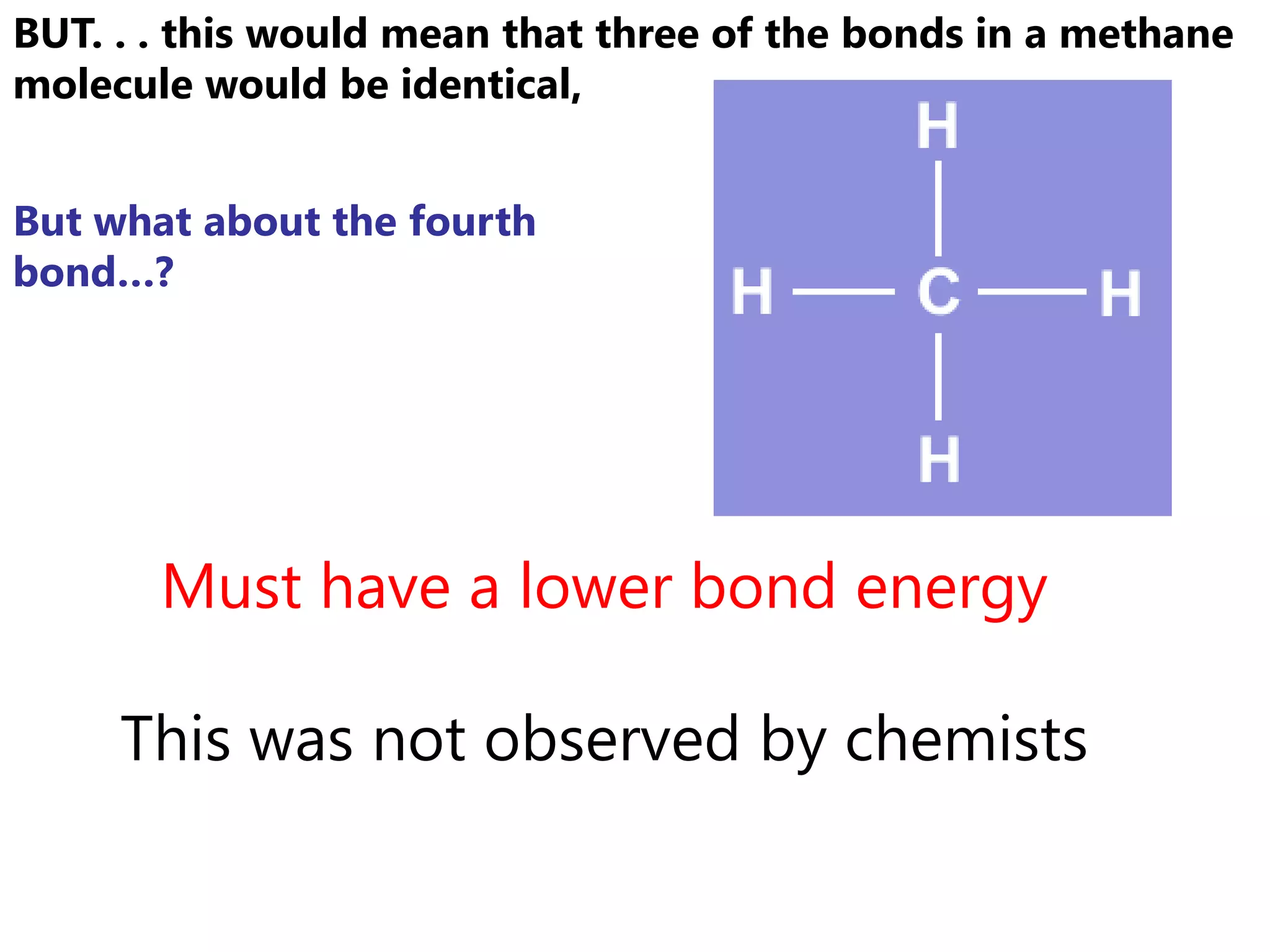
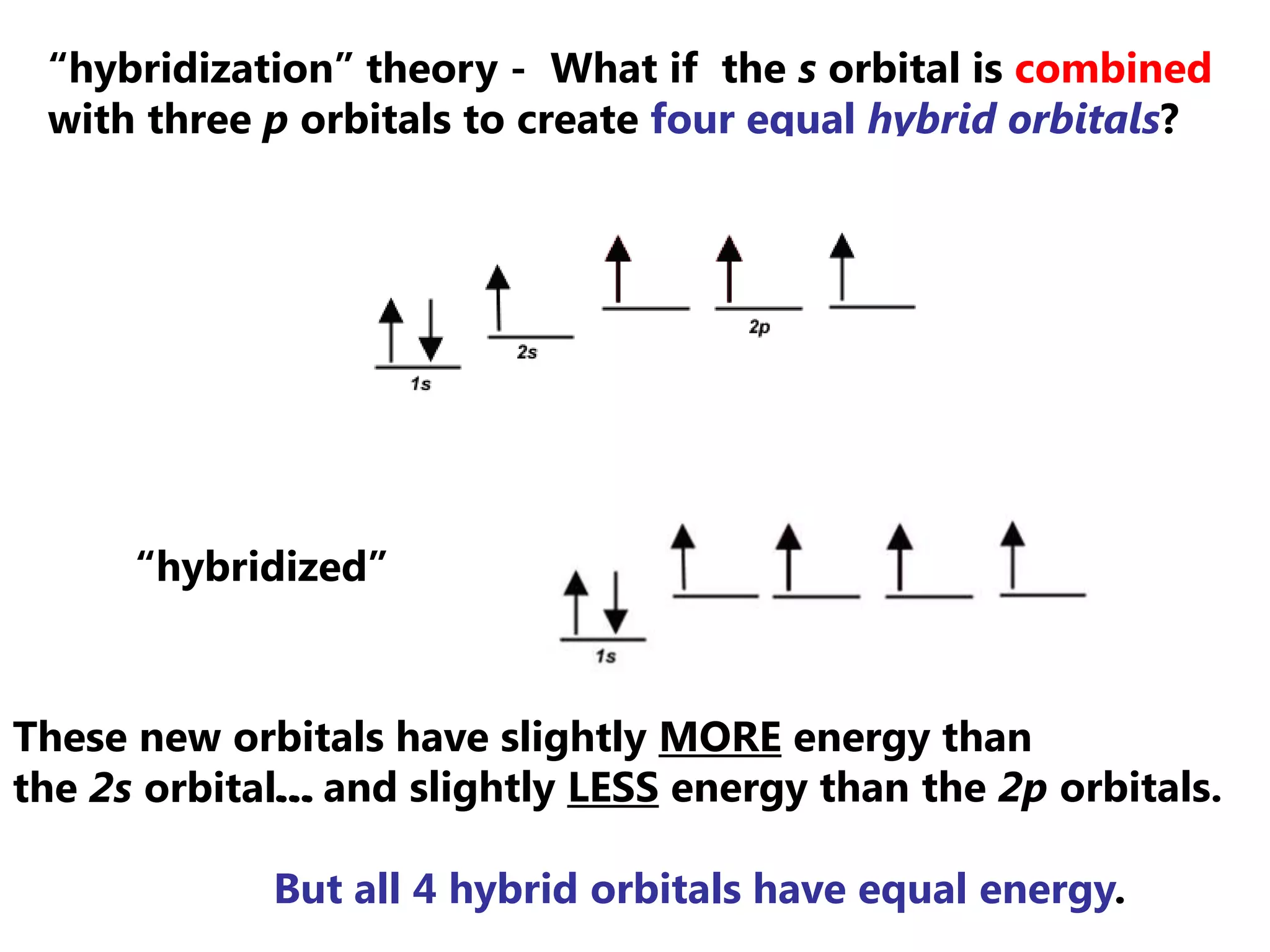
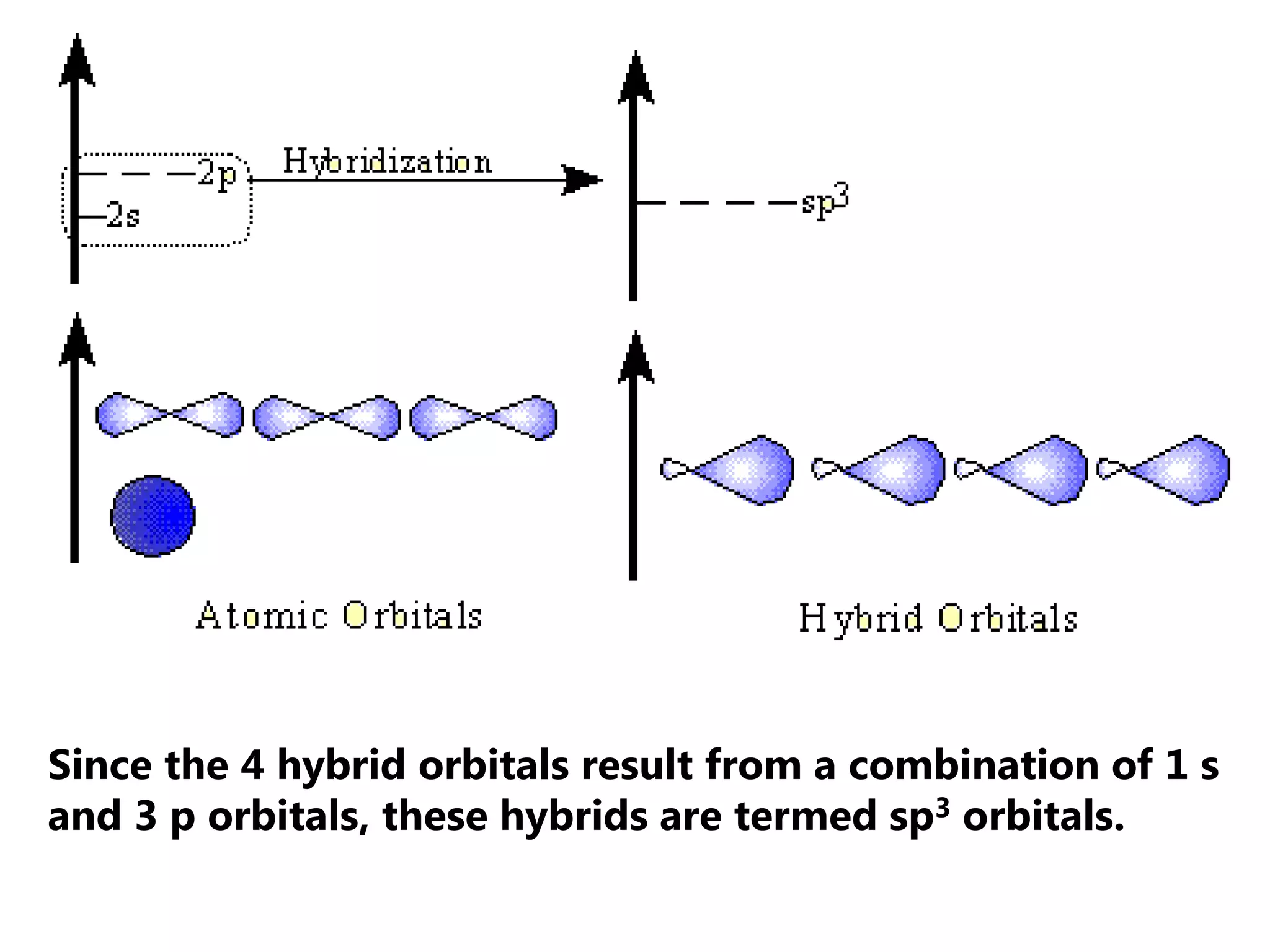
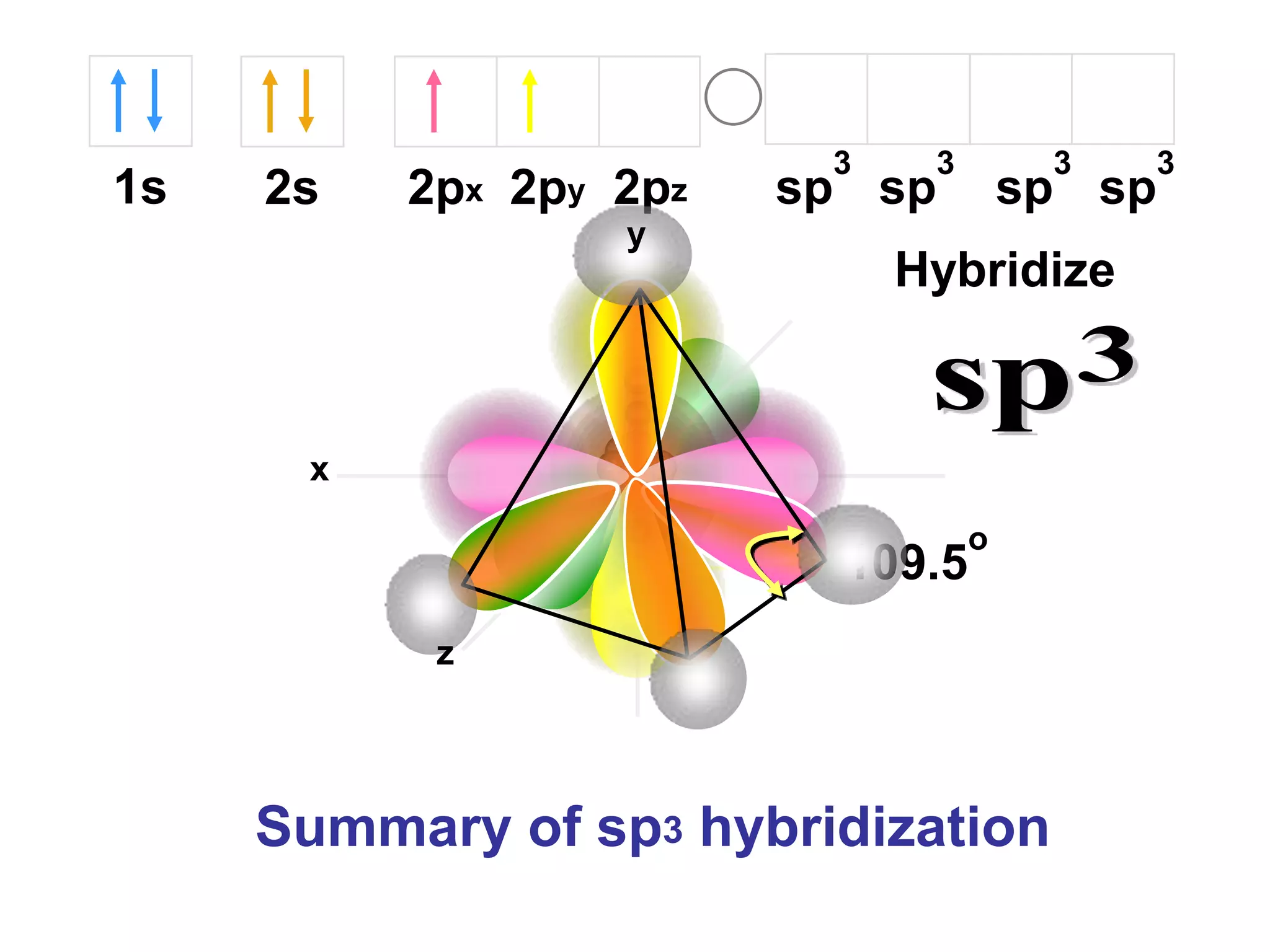
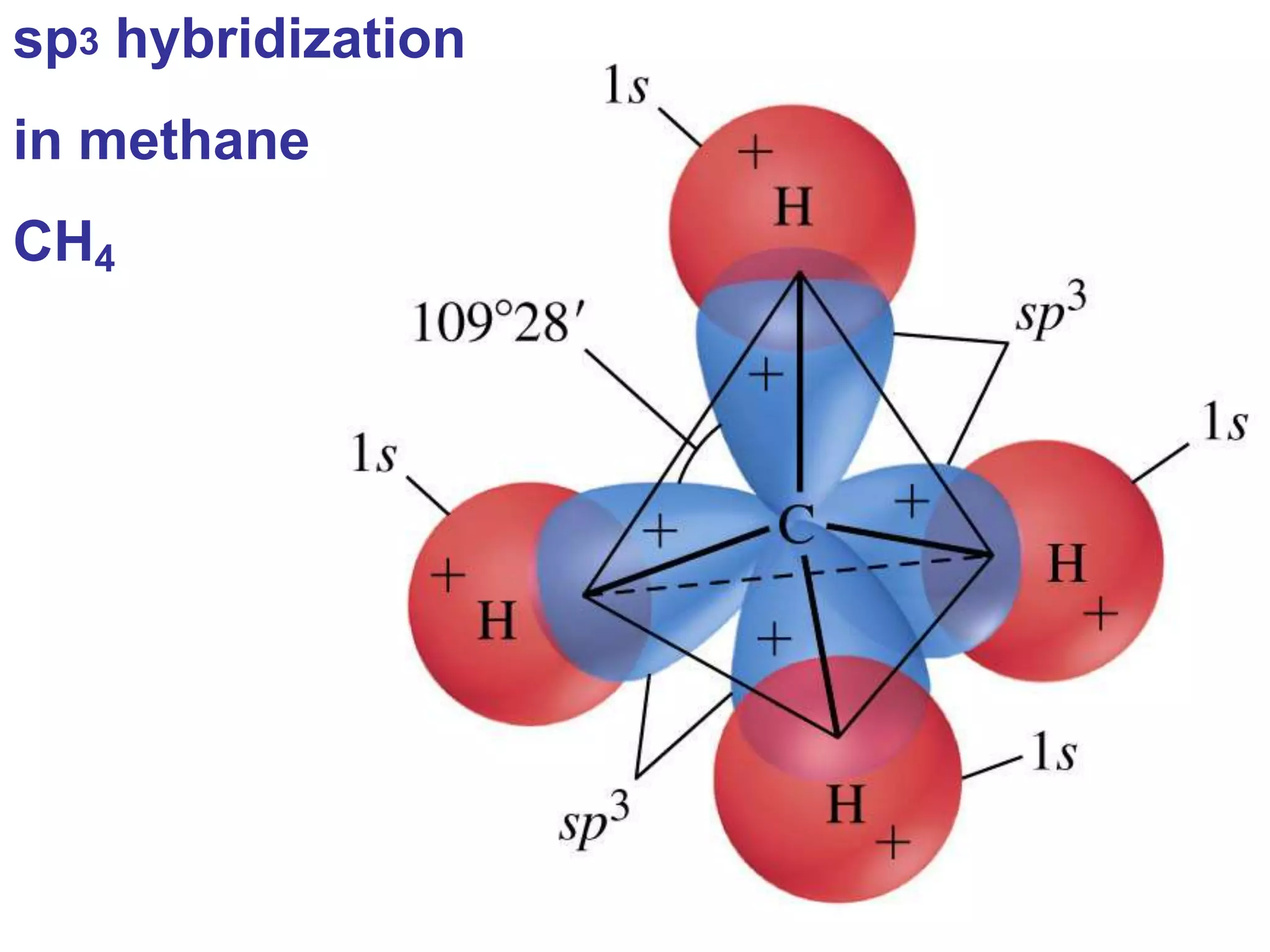
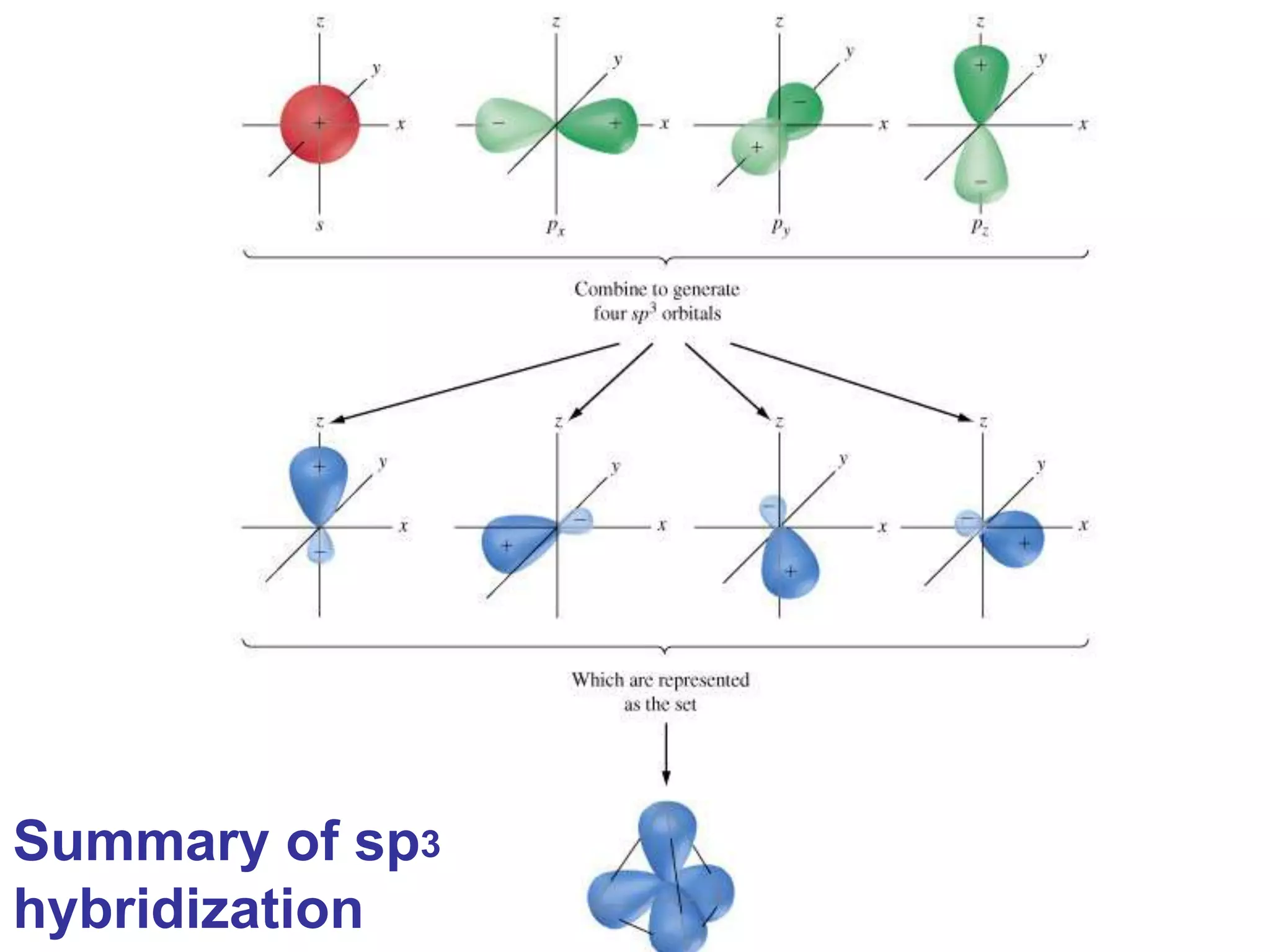
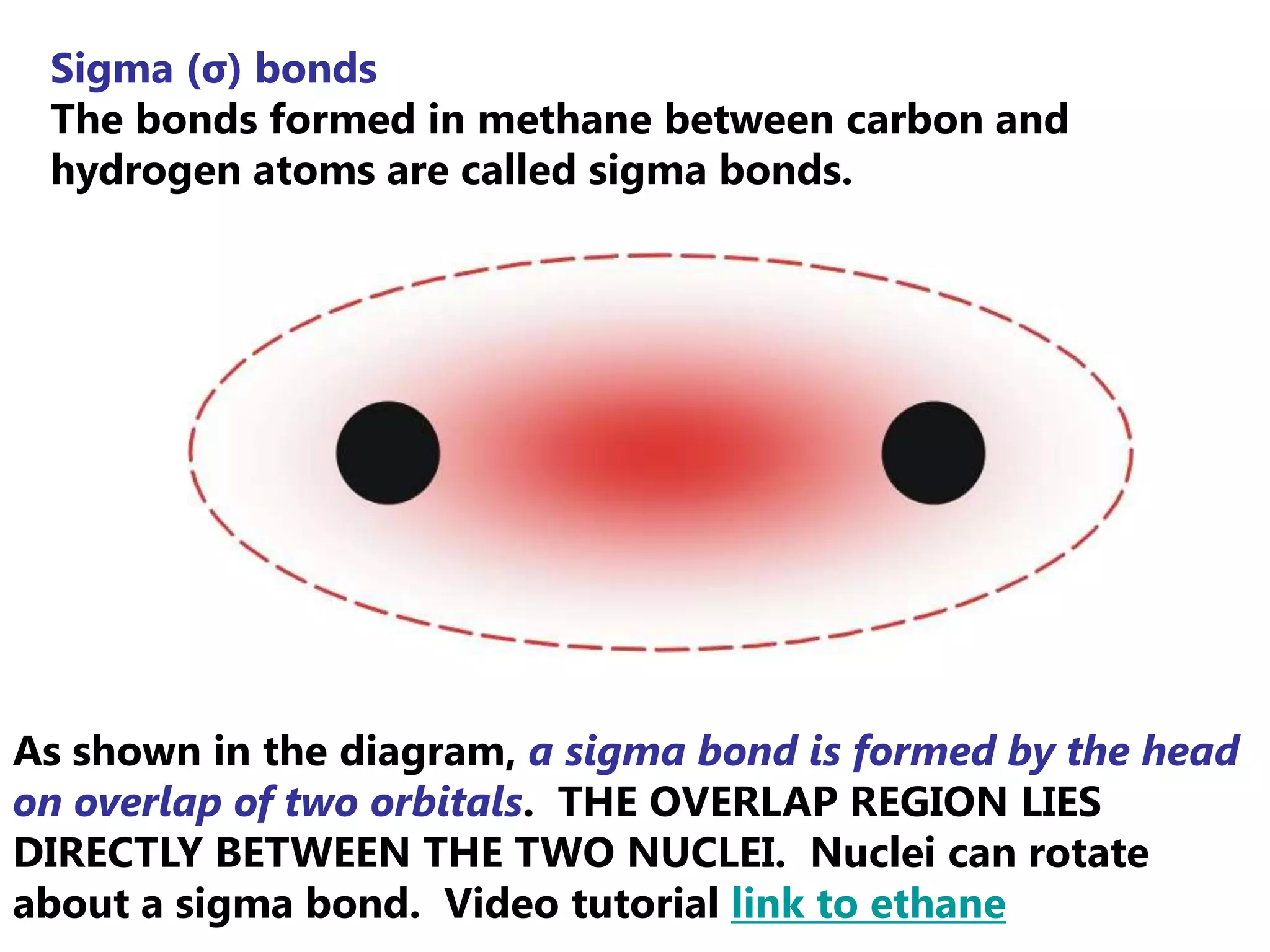
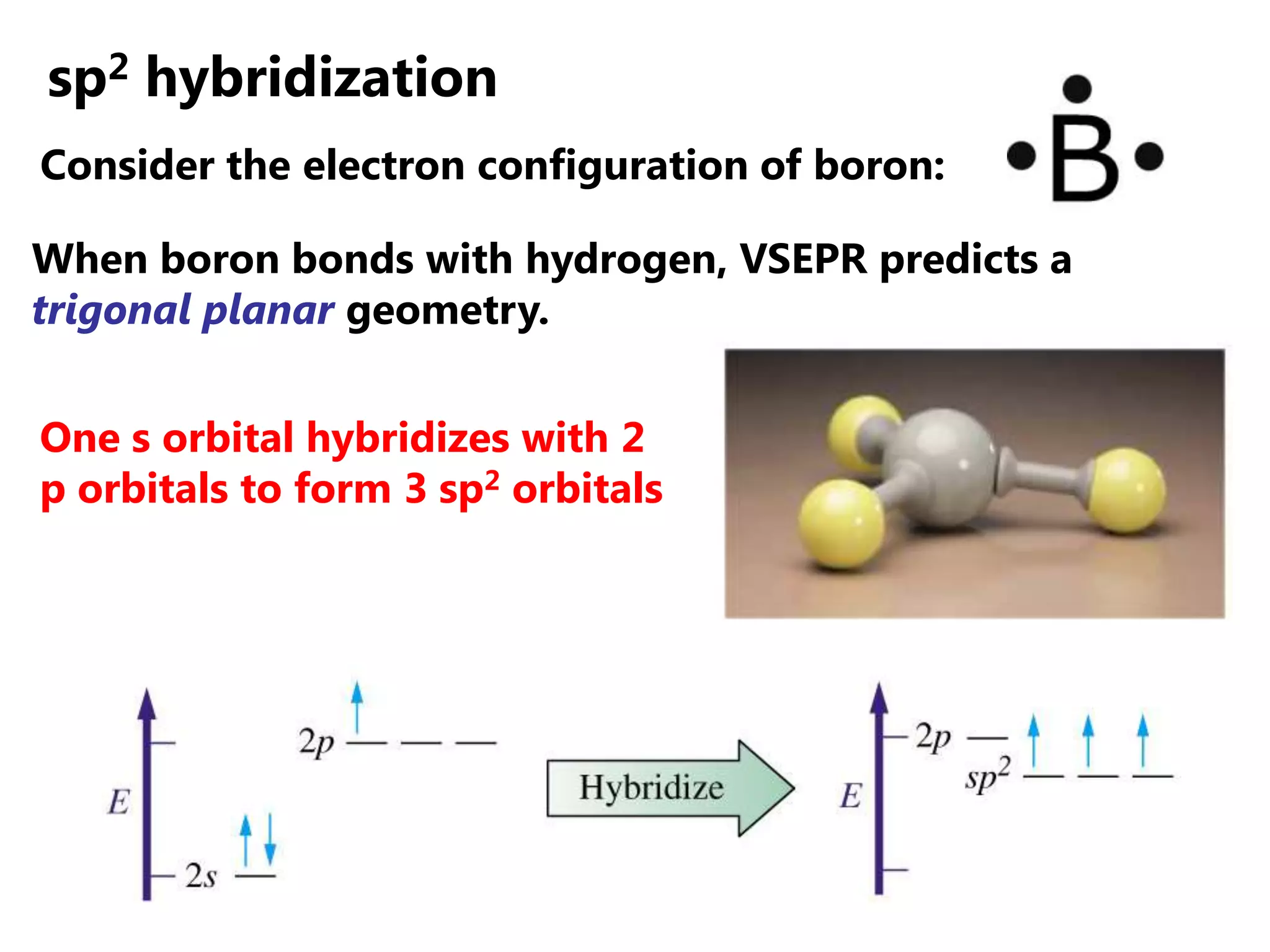
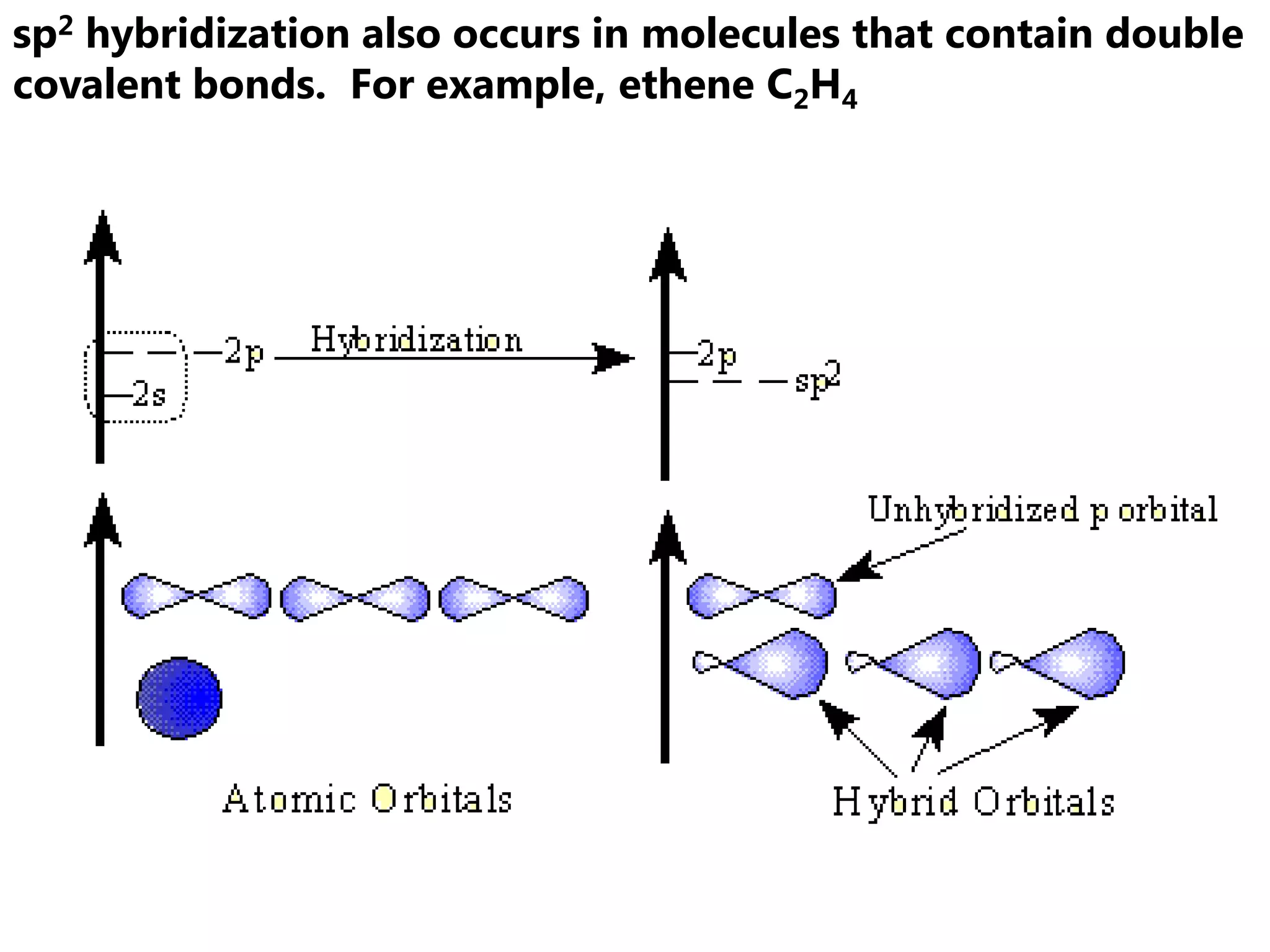
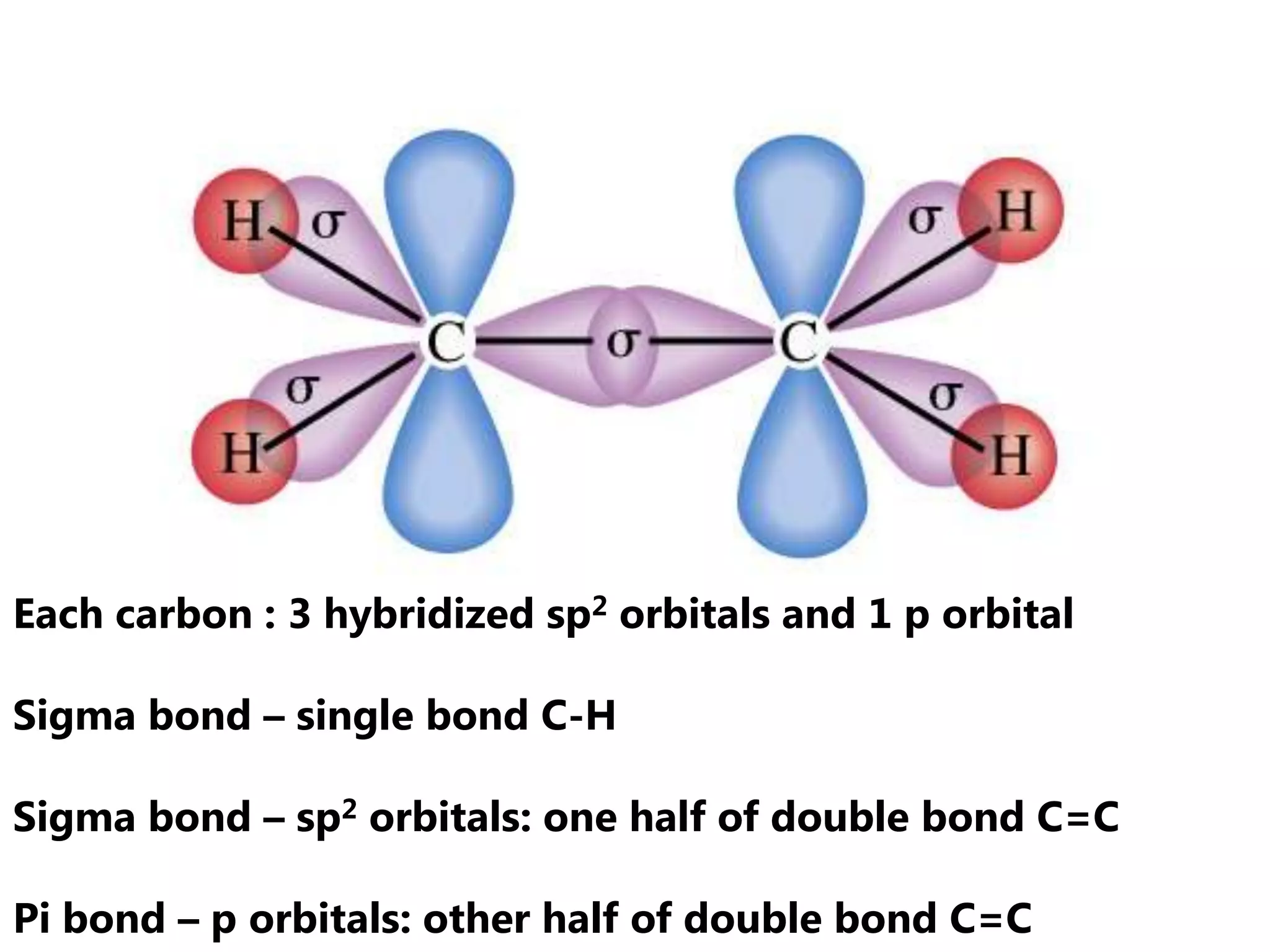
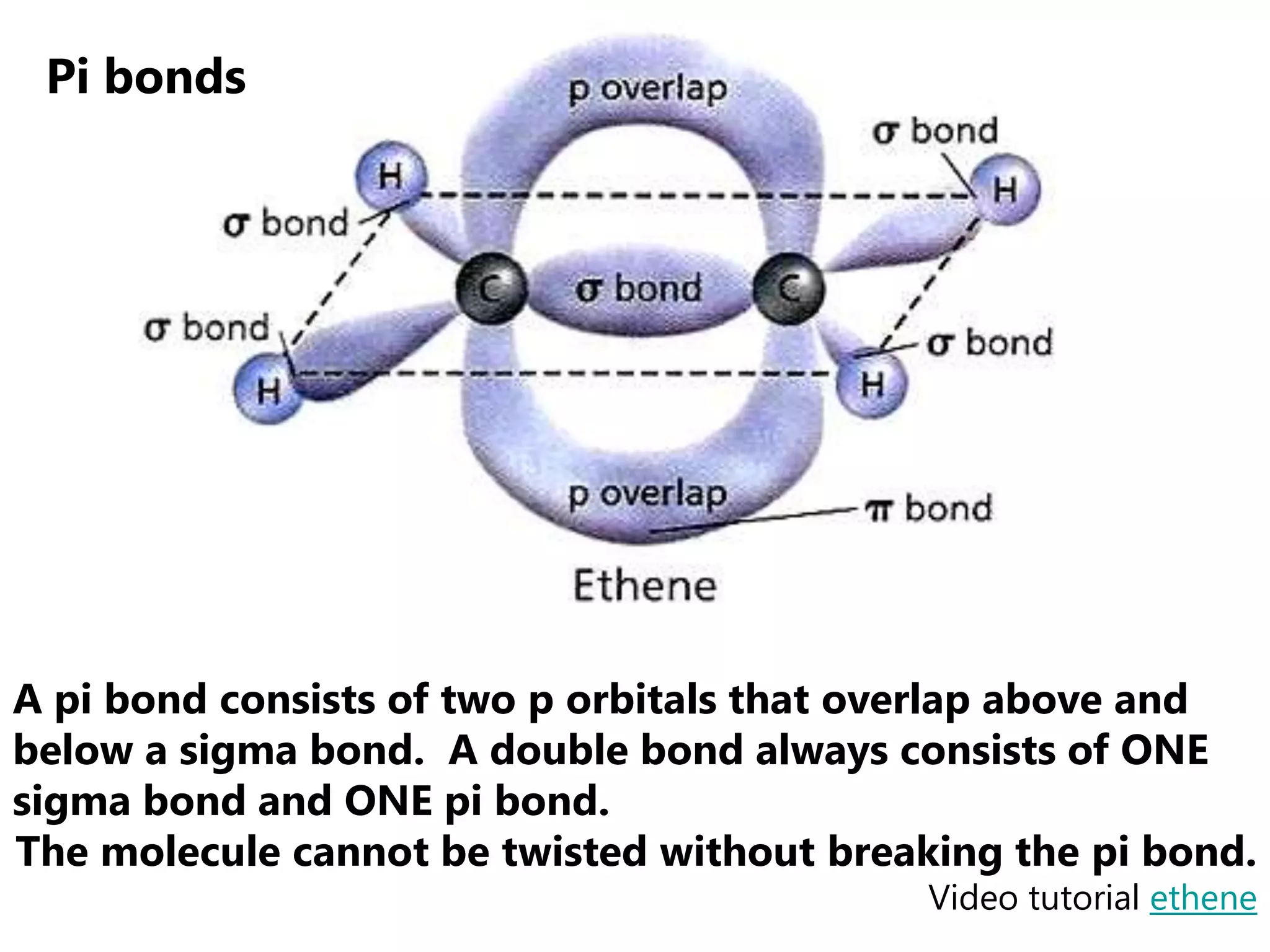
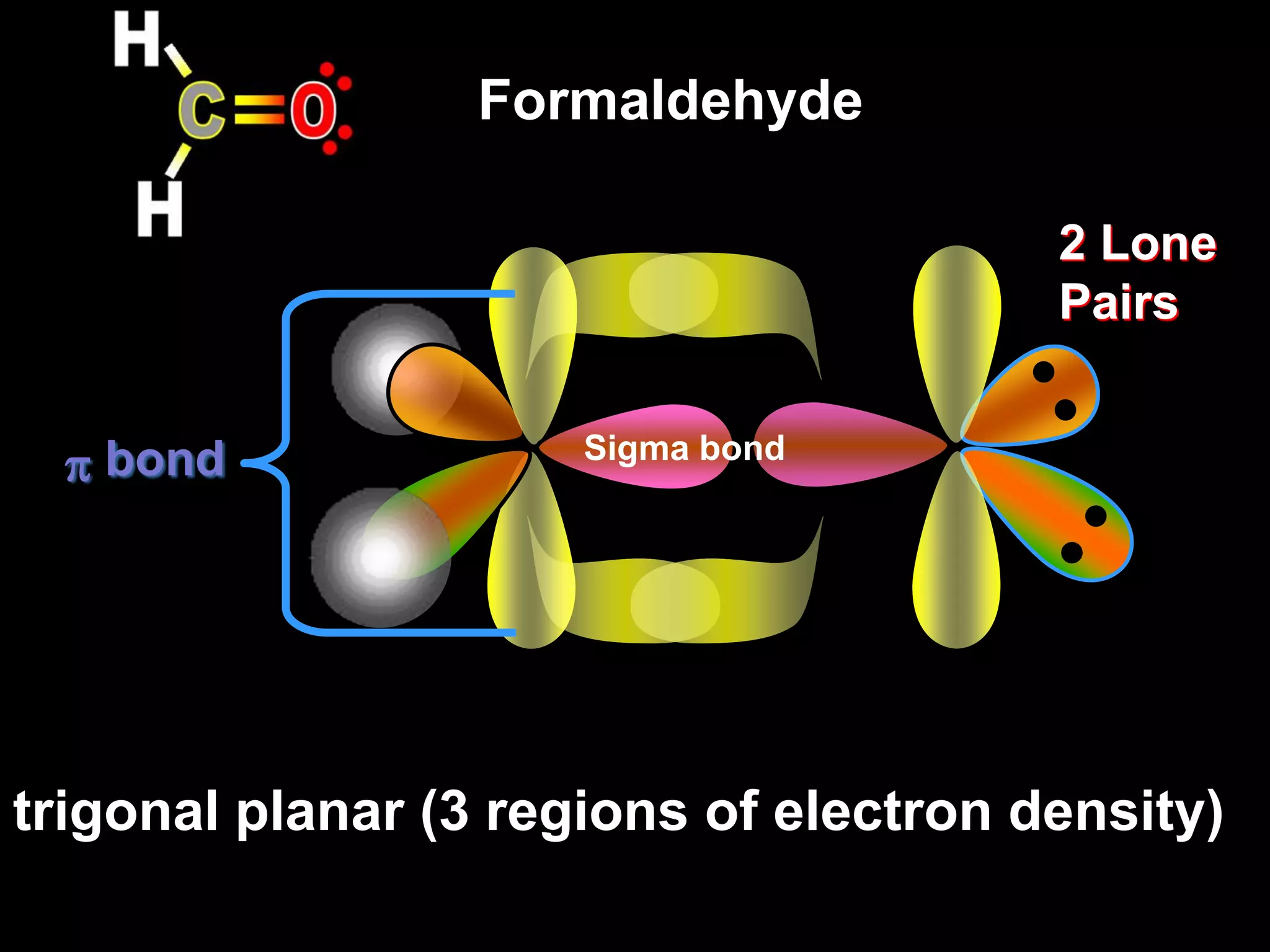
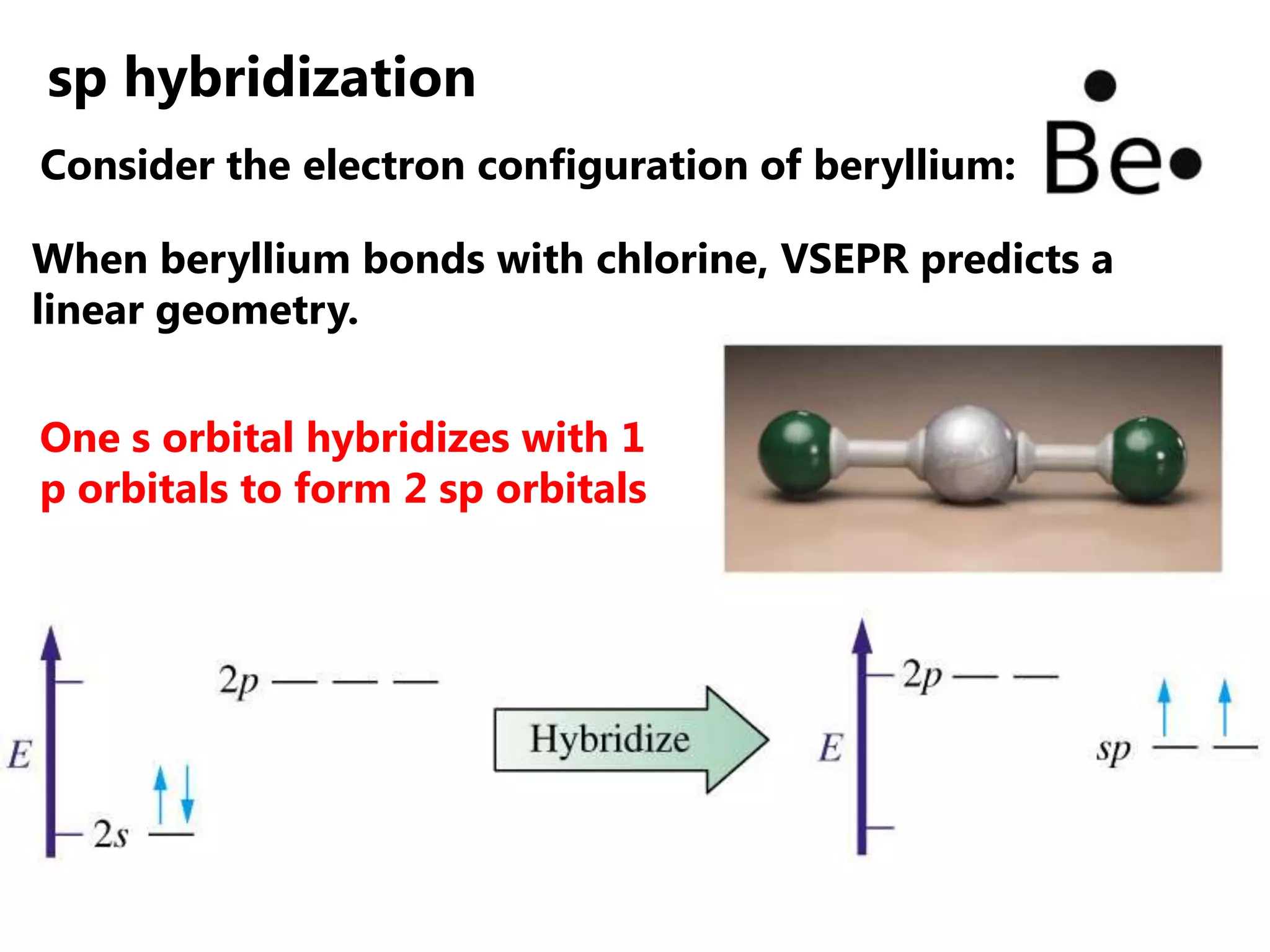
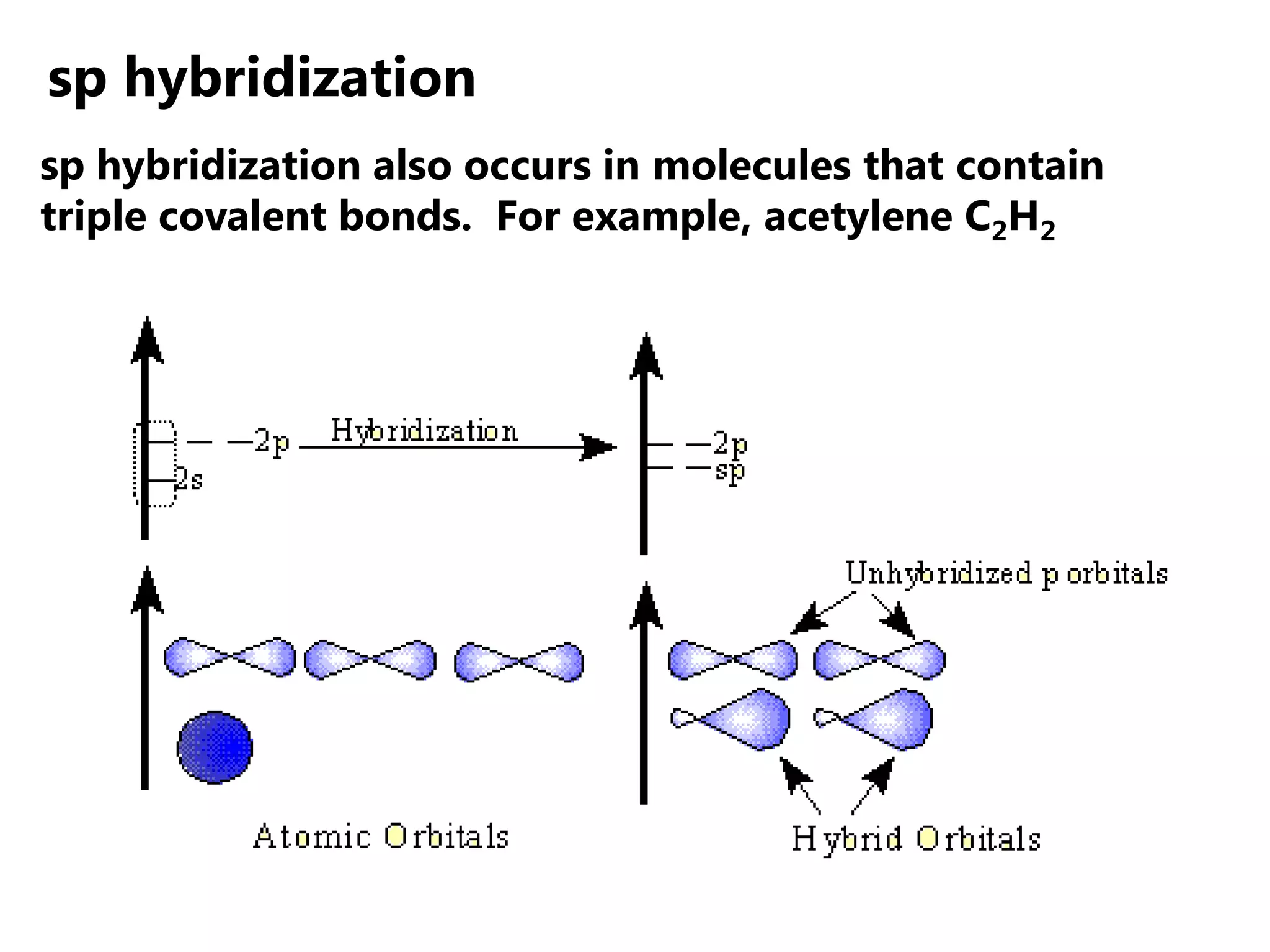
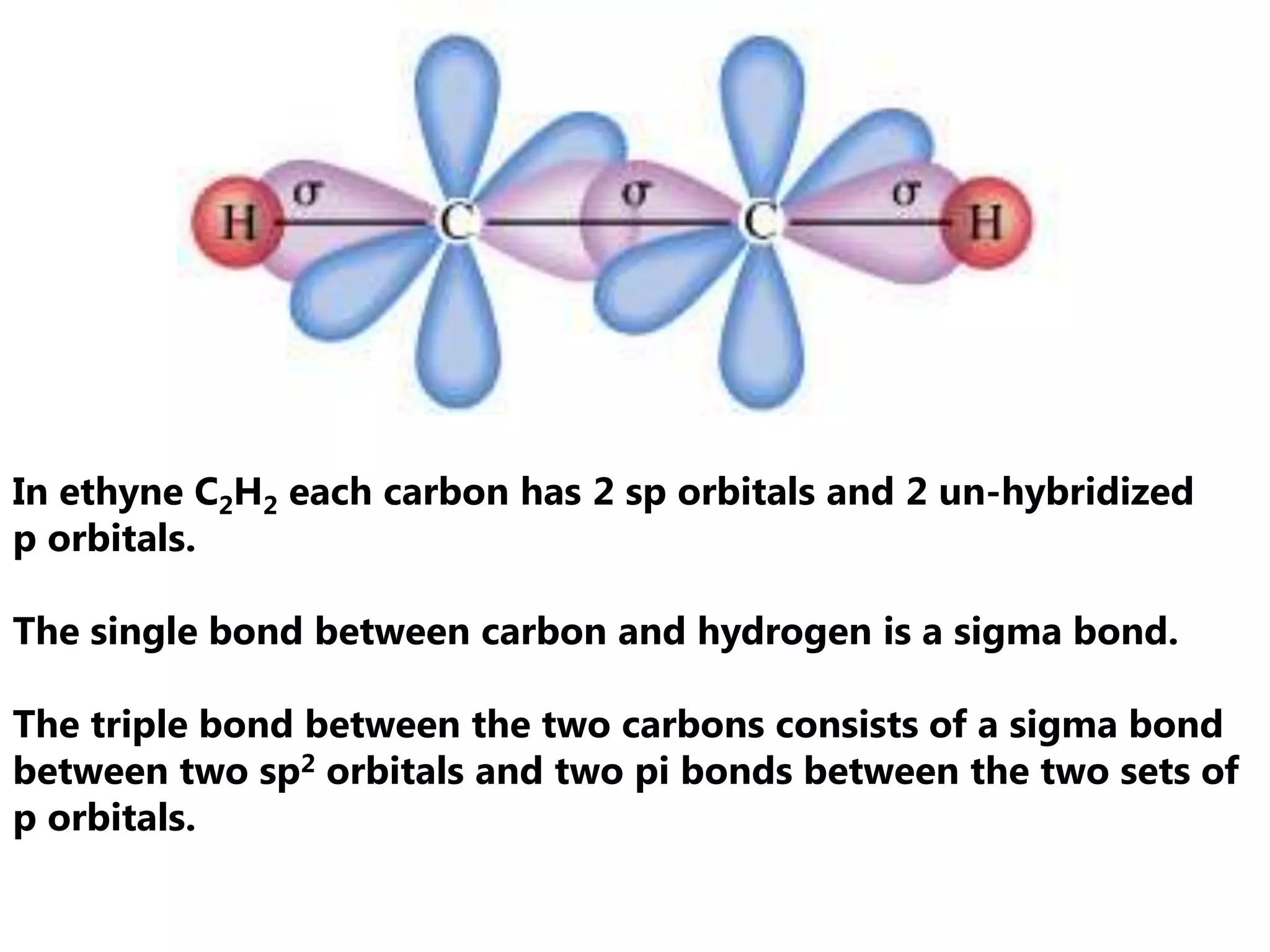
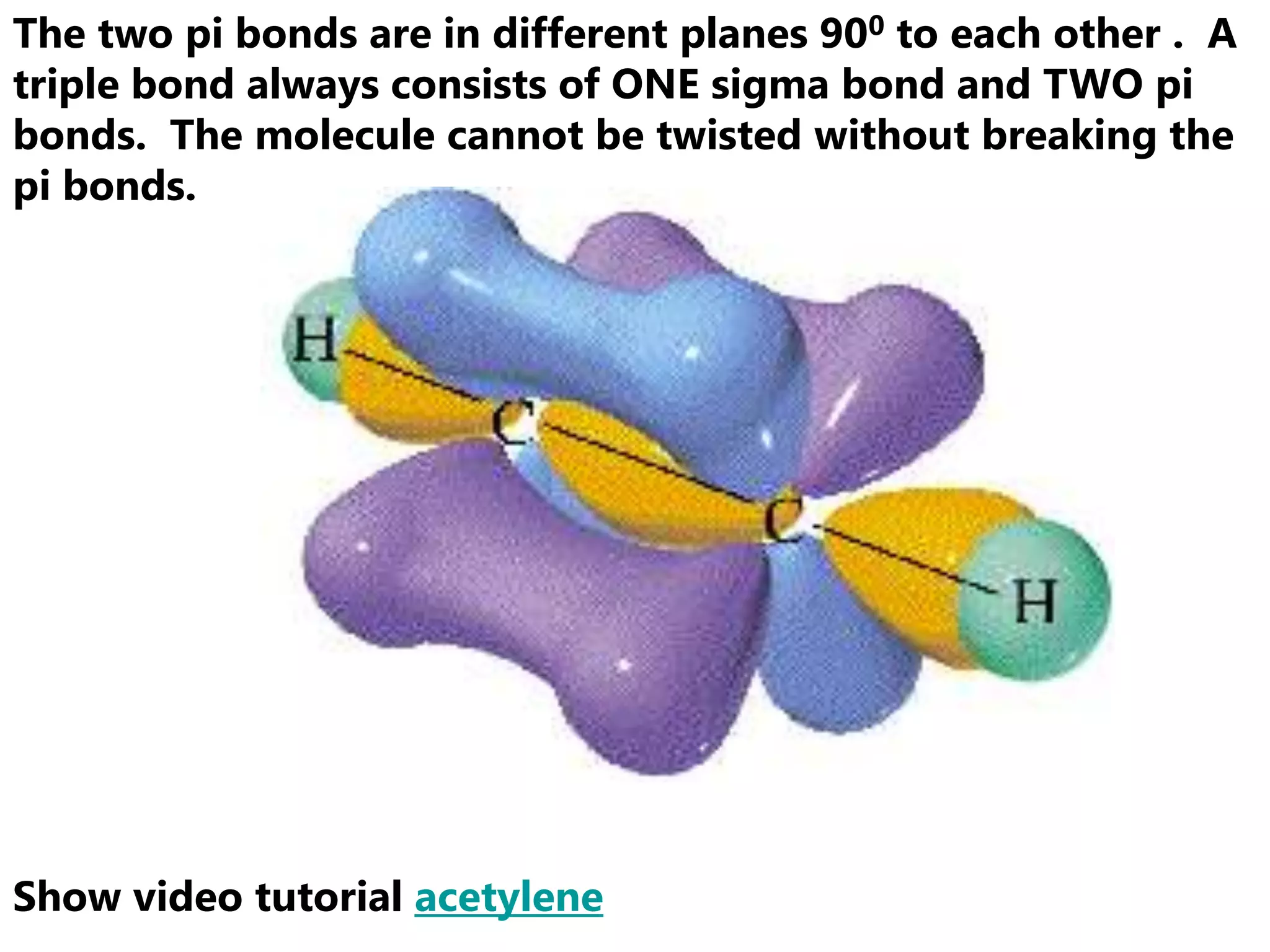
Carbon forms four equal hybrid orbitals through sp3 hybridization to allow methane to adopt its tetrahedral electron geometry. This involves one s orbital and three p orbitals combining to form four new hybrid orbitals. Sigma bonds are formed by the head-on overlap of hybrid orbitals. Sp2 hybridization with one s and two p orbitals results in trigonal planar geometries like ethene. Pi bonds in double and triple bonds involve overlap of unhybridized p orbitals above and below the sigma bond.