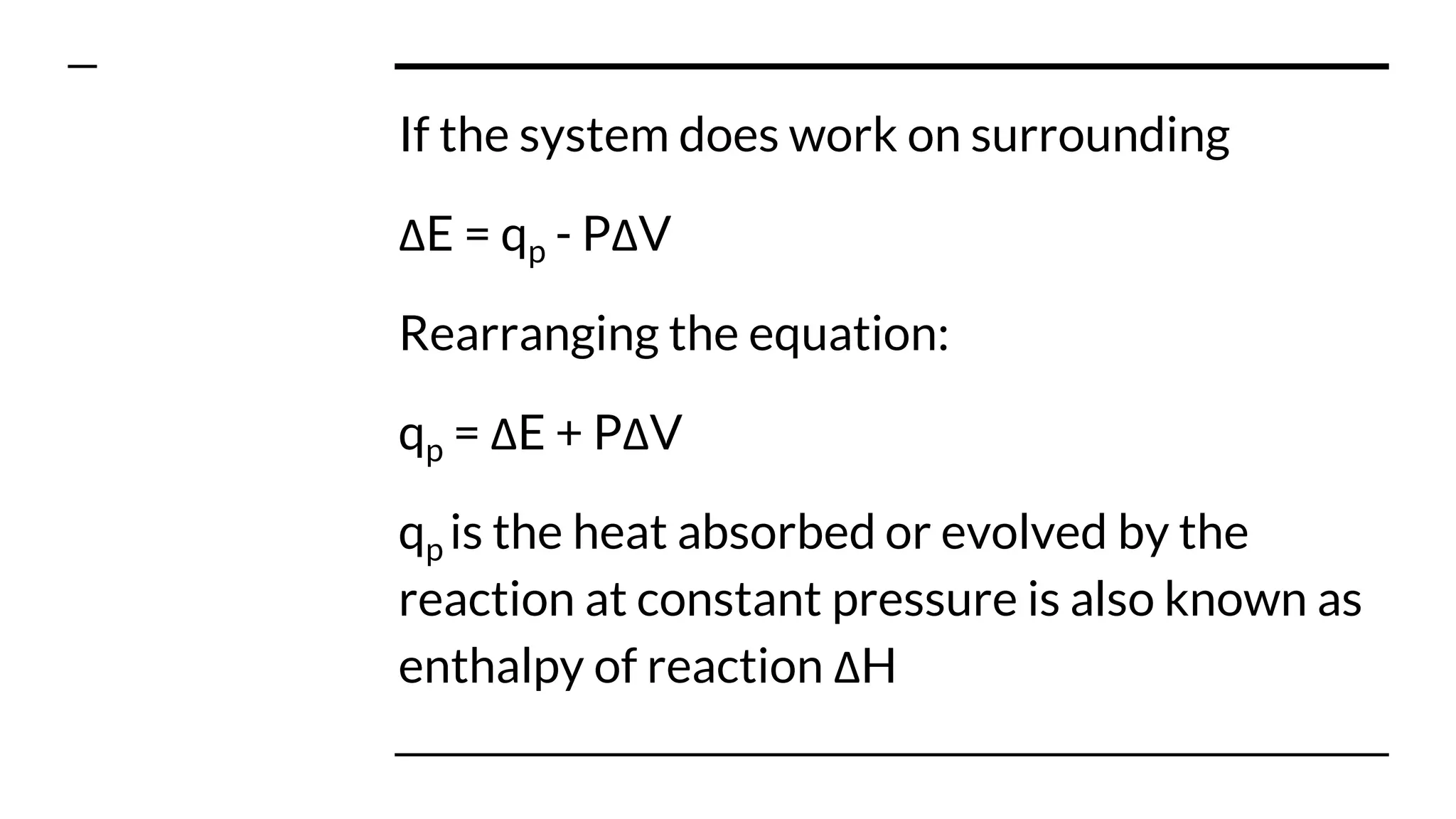
1) Enthalpy is a measure of the heat absorbed or released during a chemical reaction at constant pressure. It is equal to the change in internal energy of the system plus the product of pressure and change in volume.
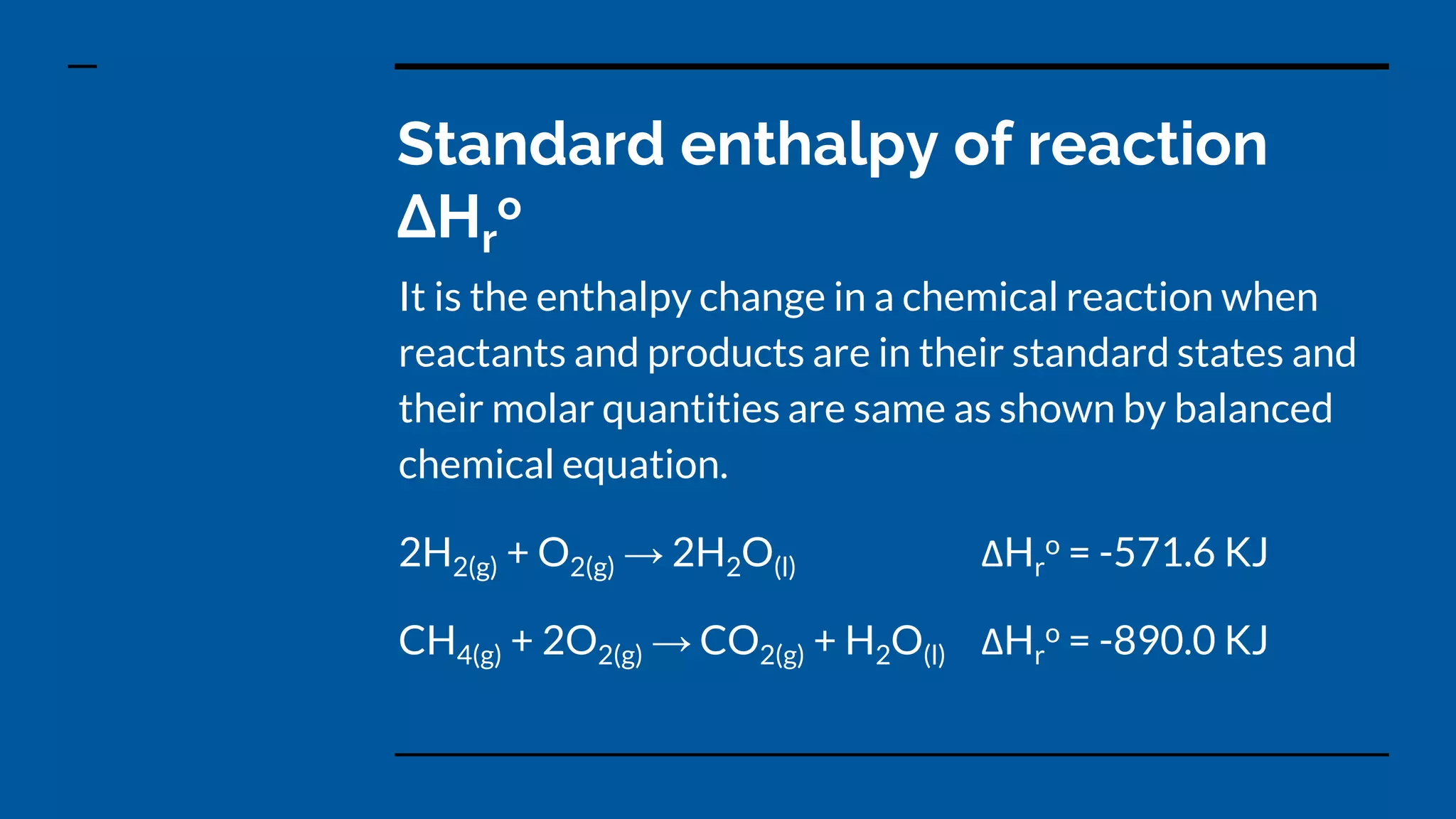
2) The standard enthalpy change of a reaction is the enthalpy change that occurs under standard state conditions of 1 atm pressure and 25°C temperature.
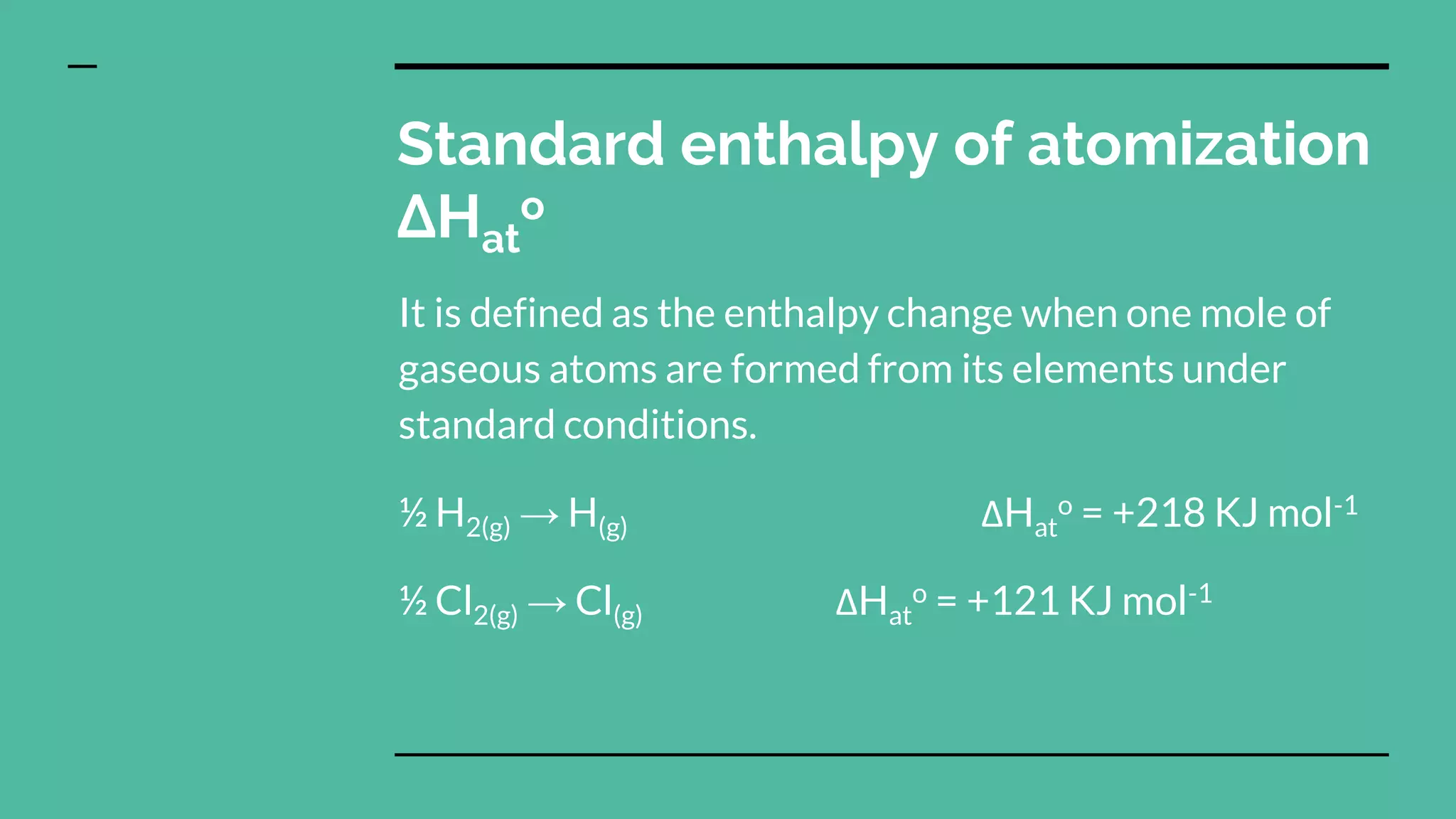
3) Standard enthalpy changes of formation, combustion, atomization, neutralization, and solution can be defined based on specific chemical processes occurring under standard state conditions.