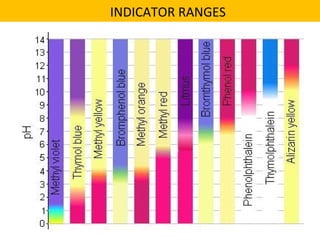
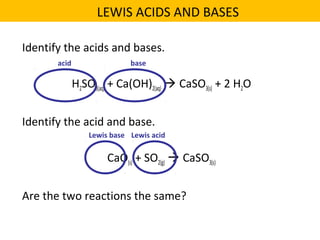
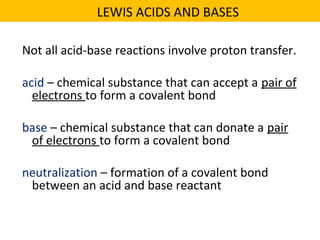
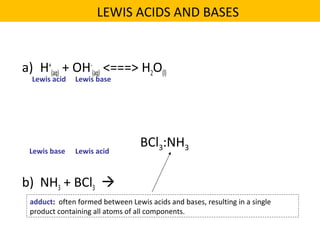
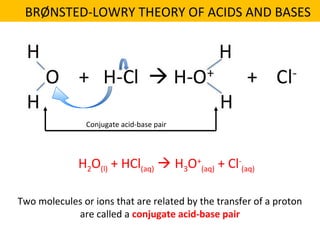
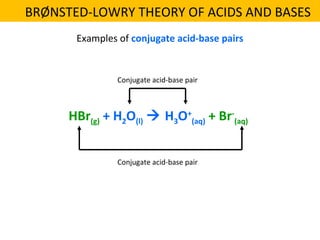
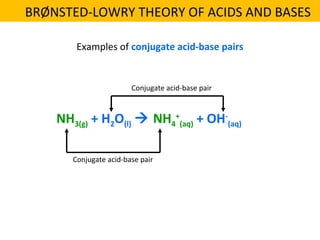
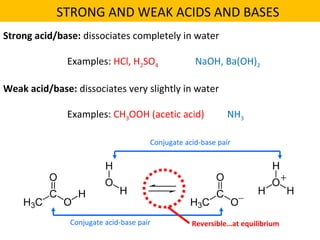
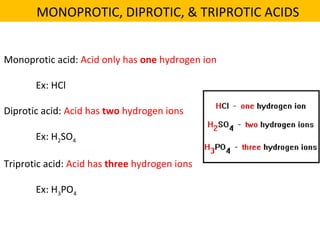
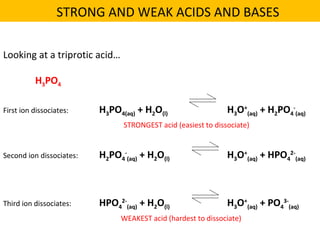
This document discusses acids and bases. It defines their key properties including reacting with metals, carbonates, conducting electricity, turning litmus paper colors, and neutralizing each other. It explains the theories of Arrhenius, Brønsted-Lowry, and Lewis on acids and bases. It also covers acid-base reactions, indicators, pH, titrations, strong/weak acids and bases, and acid-base stoichiometry.








![H2O(l) + H2O(l) H3O+
(aq) + OH-
(aq)
[H3O+
] = [OH-
] = 1.0 x 10-7
mol/L
In a neutral solution at 25ºC…
Concentration of H3O+
Concentration of OH-
pH
Negative
logarithm of…
Or
-log
Concentration
of H3O+
ions (in
mol/L)
Or
[H3O+
]
POWER OF HYDROGEN (pH)](https://image.slidesharecdn.com/22acidsbases-160608171443/85/22-acids-bases-28-320.jpg)
![Therefore pH of water = -log [H3O+
]
= -log [1.0x10-7
]
= -(-7.00)
= 7.00
POWER OF HYDROGEN (pH)](https://image.slidesharecdn.com/22acidsbases-160608171443/85/22-acids-bases-29-320.jpg)
![pH = - log [H3
O+
]
pOH = - log [OH-
]
[H3
O+] = 10 –pH
[OH-] = 10-pOH
pH + pOH = 14
Formulae involving pH:
POWER OF HYDROGEN (pH)](https://image.slidesharecdn.com/22acidsbases-160608171443/85/22-acids-bases-30-320.jpg)
![1. Calculate the pH of a solution with [HCl(aq)] = 0.75 mol/L
POWER OF HYDROGEN (pH)
HCl(aq) H+
(aq) + Cl-
(aq)
0.75mol/L 0.75mol/L Due to a 1:1 mole ratio
pH = -log [H+
(aq)]
= -log [0.75 mol/L]
= 0.12
2. Calculate the pH of a solution with [H2SO4(aq)] = 0.75 mol/L
H2SO4(aq) 2H+
(aq) + SO4
2-
(aq)
0.75mol/L 1.5mol/L Due to a 1:2 mole ratio
pH = -log [H+
(aq)]
= -log [1.5 mol/L]
= -0.18](https://image.slidesharecdn.com/22acidsbases-160608171443/85/22-acids-bases-31-320.jpg)
![pH = 14 – pOH
= 14 – (2.42)
= 11.58
3. Calculate the pH of a solution with [NaOH(aq)] = 3.8x10-3
mol/L
POWER OF HYDROGEN (pH)
NaOH(aq) Na+
(aq) + OH-
(aq)
0.0038mol/L 0.0038mol/L Due to a 1:1 mole ratio
pOH = -log [OH-
]
= -log [3.8x10-3
]
= 2.42](https://image.slidesharecdn.com/22acidsbases-160608171443/85/22-acids-bases-32-320.jpg)