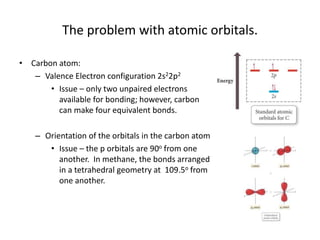
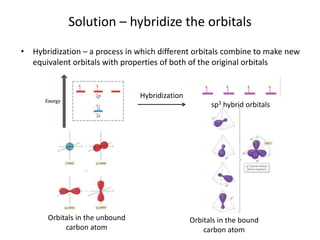
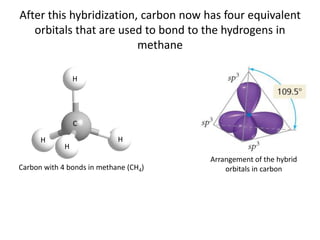
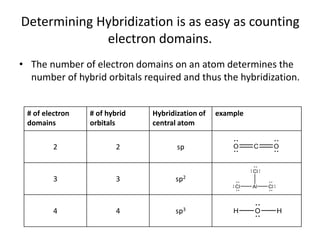
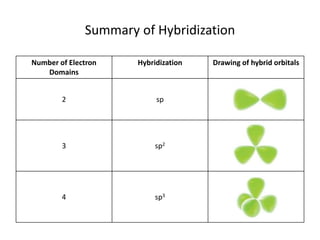
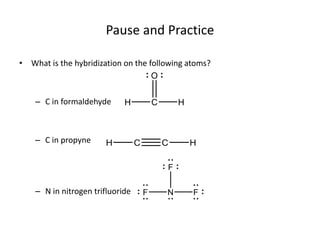
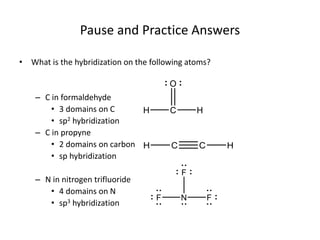
This document discusses hybrid orbital bonding theory. It explains that atomic orbitals alone cannot describe bonding in molecules like methane, where carbon forms four equivalent bonds arranged tetrahedrally. Carbon's atomic orbitals cannot account for this. The solution is that carbon's 2s and 3p orbitals hybridize to form four new equivalent sp3 hybrid orbitals oriented at 109.5 degrees, allowing carbon to form four sigma bonds to hydrogen in methane. Hybridization is determined by counting electron domains, with sp, sp2, and sp3 hybridization occurring for 2, 3, and 4 domains respectively. This model explains methane's bonding and tetrahedral structure.