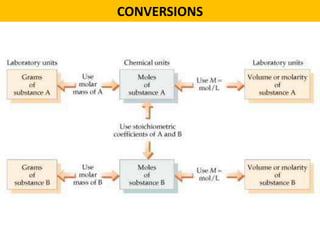
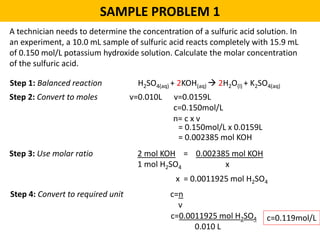
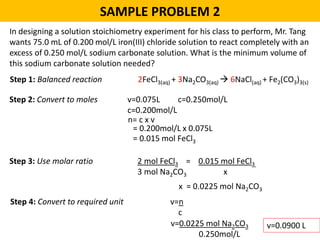
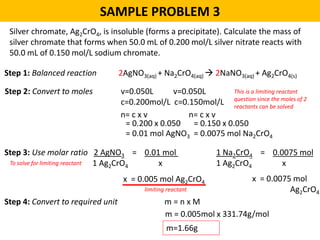
This document provides an overview of solution stoichiometry and includes 3 sample problems demonstrating how to use a balanced chemical equation to solve for unknown quantities. The general steps are: 1) Write the balanced equation, 2) Convert given amounts to moles, 3) Use the mole ratio to calculate moles of the unknown, 4) Convert moles to the required unit. Sample Problem 1 calculates molar concentration of sulfuric acid. Sample Problem 2 determines the minimum volume of sodium carbonate needed. Sample Problem 3 calculates the mass of silver chromate precipitate formed.