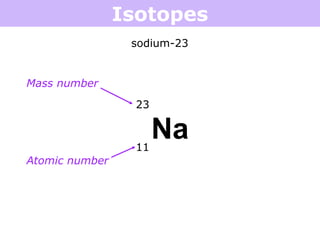
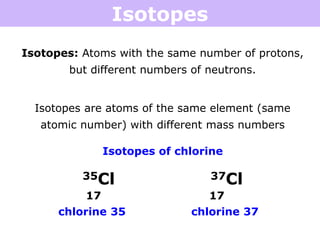
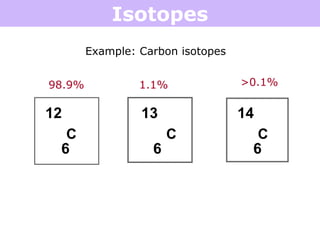
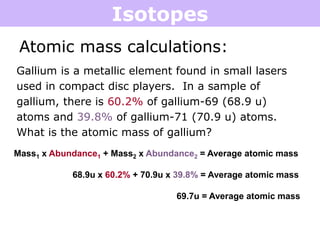
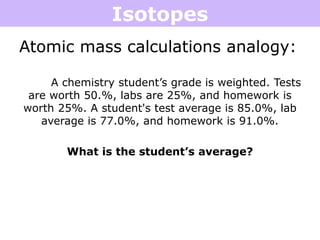
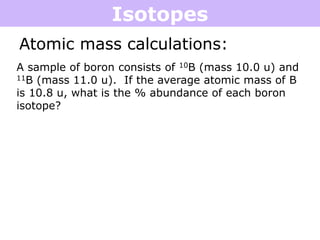
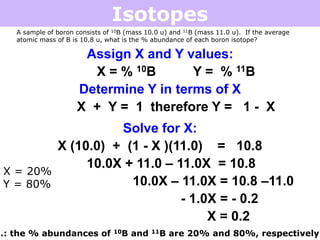
Isotopes are atoms of the same element that have different numbers of neutrons. Atoms of the same element can have different mass numbers depending on their number of neutrons. The atomic mass listed on the periodic table is an average that takes into account the relative abundances of each isotope of that element. Atomic mass calculations involve multiplying the mass and abundance percentage of each isotope and adding them together.